Benzene was derived historically from gum benzoin, also known as ‘Benjamin.’ Gum benzoin was classified as an aromatic resin. Benzene was discovered in illuminating gas by Michael Faraday, an English scientist. Mitscherich, a German chemist, coined the term benzene in 1833. Although benzene is a naturally occurring substance produced by volcanoes and forest fires and found in many plants and animals, it is also a major industrial chemical derived from coal and oil.
| Definition: It is an aromatic organic compound with C6H6 as the molecular formula. |
Benzene Chemistry Questions with Solutions
Q-1: Benzene is aromatic because it follows
- Huckel’s rule
- Aromaticity conditions
- 4n pi electron rule
- None of the above
Answer: a) Huckel’s rule
Explanation: According to the Huckel rule, compound should possess,
(i) Planarity
(ii) π electrons complete delocalisation in the ring
(iii) (4n + 2) π electrons presence in the ring where n is an integer (n = 0, 1, 2, . . .).
In the case of benzene, there are 6 pi electrons.
Hence, 4n+2 =6
This results in, n=1 (a positive value)
Because benzene follows Huckel’s rule, it is aromatic.
Q-2: Is benzene soluble in ethanol?
Answer: No
Explanation: According to the “like dissolves like” solubility principle, polar solutes can be dissolved in polar solvents and nonpolar solutes can be dissolved in non-polar solvents.
Because benzene is nonpolar and ethanol is polar, it is not soluble in ethanol.
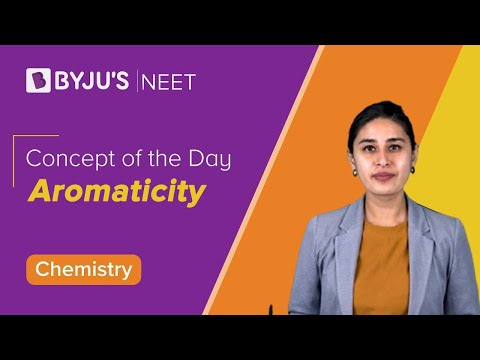
Q-3: NO2+ is formed as an electrophile during the nitration of benzene. Its shape is
- Bent
- Linear
- Angular
- Can’t predict
Answer: b) Linear
Explanation: The steric number of a particular atom can be used to determine the shape of a molecule.
We can write, SN = (number of electron lone pairs on the central atom) + (number of sigma bonds formed by the atom)
The structure of NO2+ molecule is:
It can be concluded from the structure that,
- Number of electron pairs on nitrogen =0
- Number of sigma bonds formed by nitrogen = 2
SN of nitrogen atom = 0+2=2
According to VSEPR theory, a steric number equal to 2 corresponds to hybridisation “sp” and the molecular geometry of the molecule as linear.
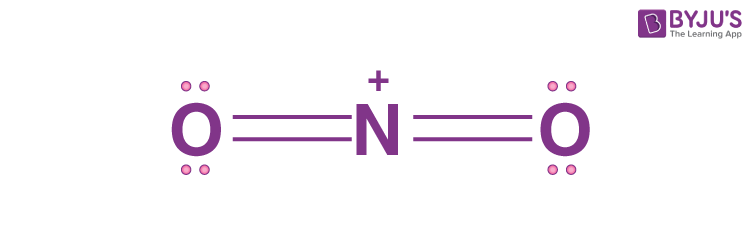
Q-4: Polymerisation of ethyne produces benzene provided the temperature is
- 573 K
- 873 K
- 673 K
- Room temperature
Answer: b) 873 K
Explanation: Cyclic polymerization occurs when ethyne passes through a red hot iron tube at 873K. Three molecules polymerise to form benzene. The reaction is shown below:
Q-5: Benzene burns with a
- Non sooty flame
- Sooty flame
- Blue flame
- Non-Luminous flame
Answer: b) Sooty flame
Explanation: Incomplete combustion of hydrocarbons due to insufficient air supply results in a sooty luminous flame (yellow). Such flames are usually shown by unsaturated or aromatic hydrocarbons. Example: Benzene
If there is sufficient air supply, a non-sooty non-luminous flame (blue) is observed as hydrocarbons are completely burned. Alkanes (saturated hydrocarbons) show such kinds of flames.
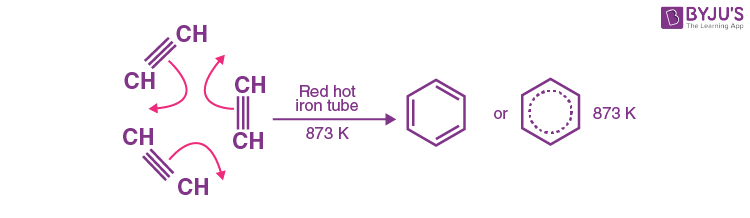
Q-6: Addition reaction is not shown by benzene because
- It is planar
- It has cyclic structure
- Double bonds are very strong
- Stabilisation due to resonance needs to be reserved
Answer: d) Stabilisation due to resonance needs to be reserved
Explanation: Because of the delocalisation of pi electrons, benzene is stable. If the benzene is subjected to an addition reaction, its aromaticity will be lost due to the formation of sp3 carbon at which delocalisation stops. As a result, it does not exhibit an addition reaction in order to avoid the loss of aromatic character.
The following reaction can help you understand in a better way:
We can clearly see that the aromatic system(highly stable) is getting converted into a non-aromatic system (less stable).
Q-7: Match the column I with column II
| Column I | Column II |
|---|---|
| 1) Benzene + Cl2 + AlCl3 → | a) Toluene |
| 2) Benzene + CH3Cl + AlCl3 → | b) Benzoic acid |
| 3) Benzene + CH3COCl + AlCl3 → | c) Chlorobenzene |
| 4) Toluene + KMnO4/NaOH → | d) Methyl phenyl ketone
e) Benzene Hexa chloride |
Answer:
| Column I | Column II |
|---|---|
| 1) Benzene + Cl2 + AlCl3 → | Chloro benzene |
| 2) Benzene + CH3Cl + AlCl3 → | Toluene |
| 3) Benzene + CH3COCl + AlCl3 → | Methyl phenyl ketone |
| 4) Toluene + KMnO4/NaOH → | Benzoic acid |
Q-8: In which of the following systems, benzene ring is not present?
- Naphthalene
- Phenanthrene
- Anthracene
- Azulene
Answer: d) Azulene
Explanation: A system that consists of a benzene ring is said to be a benzenoid system and a system without a benzene ring is said to be a non benzenoid system.
The structure of azulene is shown below:
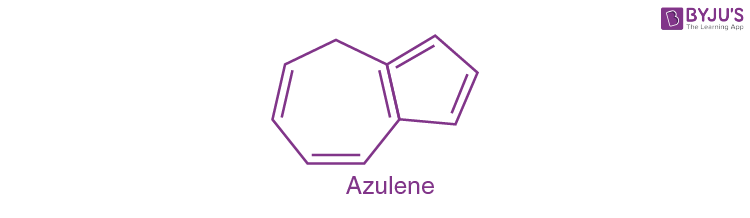
We can clearly see that there is no benzene ring present in it. Therefore a non-benzenoid system.
Q-9: Which of the following structures demonstrates that benzene has three double bonds?
- Molozonide
- Diozonide
- Trioxane
- Triozonide
Answer: d) Triozonide
Explanation: Triozonide is formed when benzene undergoes ozonolysis. Ozonolysis is an organic chemical reaction in which ozone is used to cleave the unsaturated bonds of alkenes.
Because benzene contains three double bonds, it forms a triozonide.
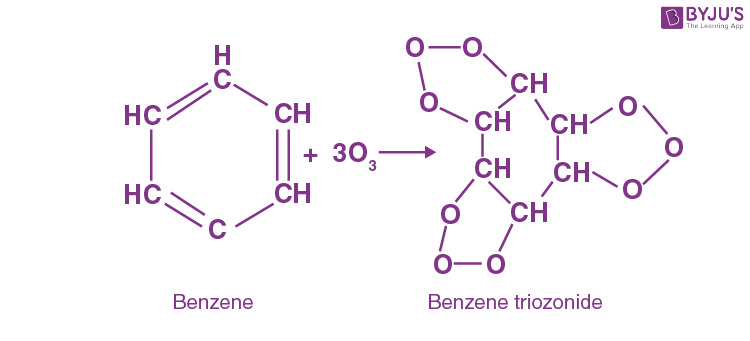
Q-10: Give the chemical equation for the combustion of benzene.
Answer: When heated in air, benzene emits a sooty flame and produces CO2 and H2O. This type of reaction is known as a combustion reaction, and it is depicted below:
C6H6 + 15/2O2 → 6CO2 + 3H2O
Q-11: State three important uses of benzene.
Answer: Benzene has a wide range of applications:
- Benzene is a common solvent in many industrial, commercial, and research applications. Manufacturers use benzene-containing products as solvents at various stages of production, and it is used in the production of chemical and plastic products. Resins, synthetic products such as nylon, Styrofoam are a few examples
- Many people and manufacturers use benzene as a fuel because of its high octane number and natural availability. It has been used as a gasoline additive to help fuel burn more efficiently.
- Most products used in the printing industry contain benzene. There are products that contain this chemical and are specifically used for cleaning printing equipment, making it last longer and function better.
Q-12: What observations did Kekule consider when assigning the structure to benzene?
Answer: Benzene was discovered to be a stable molecule that forms a triozonide, indicating the presence of three double bonds. Benzene was also discovered to produce only one monosubstituted derivative, indicating that all six carbon and six hydrogen atoms in benzene are identical. On the basis of this observation, August Kekulé proposed the cyclic arrangement of six carbon atoms with alternate single and double bonds and one hydrogen atom attached to each carbon atom in 1865.
Q-13: The carbon-carbon double bond distance in benzene is intermediate between that of C-C single and double bond distance due to
- Presence of pi electrons
- Resonance
- Its Aromatic nature
- P-orbitals
Answer: b) Resonance
Q-14: When phenol is treated with _________, it produces benzene.
Answer: Phenol is reduced to benzene by passing its vapours over heated zinc dust.
Q-15: Which structure is isoelectronic and structurally equivalent to benzene? What is the common name for it?
Answer: Borazole, or borazine, is a polar inorganic compound with the chemical formula B3H6N3. The three NH units and three BH units alternate in this cyclic compound. With benzene, the compound is isoelectronic and isostructural. As a result, borazine is also known as “inorganic benzene.”
Practise Questions on Benzene
Q-1: Which compound is formed when benzene reacts with three chlorine molecules under ultraviolet light?
- Hexachlorocyclohexane
- Gammaxane
- Hexa chloro benzene
- 1,3,5-trichloro benzene
Q-2: Which of the following name reactions uses benzene as a starting material?
- Etard reaction
- Gattermann koch reaction
- Gattermann reaction
- Sandmeyer reaction
Q-3: The overlapping of orbitals is of type ________ in benzene.
- sp-sp
- sp2-sp2
- p-p
- sp3-sp2
Q-4: How does benzene and PAHs cause toxicity?.
Q-5: How can you prepare a haloarene from benzene?
Click the PDF to check the answers for Practice Questions.
Download PDF
Recommended Videos
Resonance in Benzene

Aromaticity