What is Corrosion?
Corrosion is a natural process in which a refined metal is converted to a more chemically stable form, such as oxide, hydroxide, or sulphide. It is the gradual decomposition of materials (usually metals) as a result of chemical and/or electrochemical reactions with their surroundings.
Table of Contents
Corrosion is the gradual deterioration of metals caused by the action of air, moisture, or a chemical reaction (such as an acid) on their surface. Rusting of iron, or the forming of a brown flaky material on iron objects when exposed to moist air, is the most common example of metal corrosion.
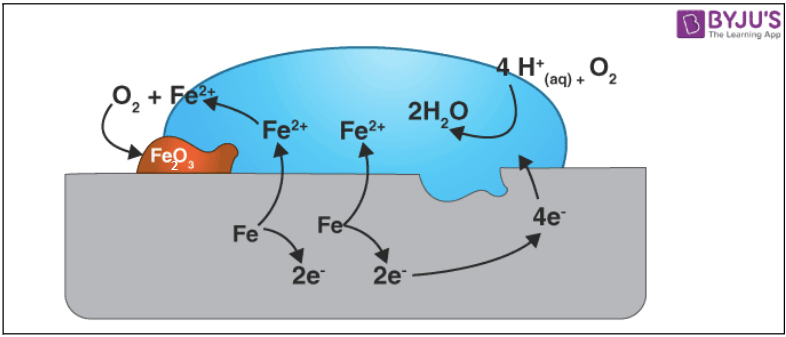
As metals are exposed to the elements, they react with the air or water in the atmosphere to produce unwanted compounds. Corrosion is the term for this operation. The atmosphere attacks the least active metals, such as gold, platinum, and palladium.
Fe (s) + O2 (g) + xH2O (l) → Fe2O3.xH2O (s)
Corrosion Explanation
Corrosion occurs when a refined metal is naturally converted to a more solid form, such as oxide, hydroxide, or sulphide, causing the substance to deteriorate. Corrosion can cause natural and historic monuments to deteriorate significantly, as well as increase the likelihood of catastrophic equipment failures. Corrosion is caused by air pollution, which is getting worse around the world.
Alkali metals, such as sodium, must be contained in oil because they corrode rapidly. Roofs are made of less reactive metals like lead and copper. Lead corrodes to form a white lead oxide or carbonate, while copper (Cu) corrodes to form a simple green carbonate. When metal reacts with another element, such as oxygen, hydrogen, electricity, or even dirt and bacteria, it corrodes. Corrosion may also occur when metals, such as steel, are subjected to excessive stress, which causes the material to crack.
Recommended Videos
Corrosion of Metals

Commercial Cells and Corrosion

Types of Corrosion
Corrosion is a natural process that transforms a refined metal into a more chemically stable form like oxide, hydroxide, or sulphide. It refers to the gradual decomposition of materials (usually metals) due to chemical and/or electrochemical reactions with their surroundings.
-
-
-
- Crevice Corrosion – Crevice corrosion is corrosion that occurs in confined spaces where the working fluid’s access to the atmosphere is limited. Crevices are the common name for these spaces. Another damaging form of localized corrosion is crevice corrosion. It typically occurs in areas where free access to the external environment is limited, such as under deposits. Contact of metals with metals or metals with non-metals, such as gaskets, couplings, and joints, causes crevice corrosion.
- Galvanic Corrosion – Galvanic corrosion is most often seen in galvanized iron, which is a zinc-coated sheet of iron or steel. The underlying steel is not attacked even though the protective zinc coating is broken. Galvanic corrosion, also known as “dissimilar metal corrosion” or “electrolysis,” occurs when two dissimilar materials are combined in a corrosive electrolyte and cause corrosion harm. It happens when two or more dissimilar metals come into electrical contact when submerged.
-
-

-
-
-
- Uniform Corrosion – Uniform corrosion is also described as corrosion that occurs at a consistent rate across an exposed metal surface. The primary cause of uniform corrosion of steel and other metals and alloys in the natural environment is oxygen. Rusting, silver tarnishing, nickel fogging, and high-temperature oxidation are all examples of uniform corrosion. For handling chemical media, the rate of uniform corrosion is usually expressed in IPY (inches penetration per year) and/or (MDD) milligrams per square decimetre per day.
- Pitting Corrosion – Pitting corrosion, also known as pitting, is a type of localized corrosion that results in the formation of small holes in metal. One of the most popular coating methods for pitting corrosion protection is zinc phosphate priming. Zinc phosphate primers, for example, are specially developed to increase corrosion resistance. Zinc spray metallizing is a highly effective anti-corrosion method.
-
-
How to Prevent Corrosion of Metals?
When corrosion has been discovered, the only sure way to repair it is to remove it. Abrasion, the details of which depend on the metallurgy of the corroded part, followed by a corrosion inhibitor, such as zinc-chromate primer, another primer, and finally paint, will eliminate light surface corrosion.
Metals can be protected from corrosion by applying one of the following coatings to their surfaces:
-
-
-
- By plastering the surface with oil, grease, paint, or varnish.
- By coating/depositing a thin film of some other non-corroding metal.
-
-
Paints can prevent corrosion by altering the anodic reaction; however, the pigment must be metallic, simple, or soluble in order for this to happen. Paint films, in general, provide protection due to their high electrolytic resistance; they readily acquire a charge and, as a result, are relatively impervious to ions.
Water and oxygen absorption from the atmosphere to the metal surface can be slowed by coatings. Corrosion is slowed as a result of this. The rate of diffusion of corrosion products from the metal surface through the paint film can be slowed by the paint film. Corrosion is also slowed as a result of this.
The iron alloy steel is coated with a less active metal, such as tin, for anodic protection. Tin does not corrode, so as long as the tin coating is in place, the steel will be covered. Since the steel becomes the anode of an electrochemical cell, this approach is known as anodic safety.
Frequently Asked Questions – FAQs
What is the process of corrosion?
Corrosion is the mechanism by which metals that have been processed revert to their normal oxidation states. This is a reduction-oxidation reaction in which the metal is oxidized by its environment, which is usually oxygen in the air. This reaction is favoured both electrochemically and spontaneously.
How do you treat corrosion?
When corrosion has been discovered, the only sure way to repair it is to remove it. Abrasion (the details of which depend on the metallurgy of the corroded part), followed by a corrosion inhibitor, such as zinc-chromate primer, another primer, and finally paint, will eliminate light surface corrosion.
How is metal corrosion prevented?
Corrosion can be avoided by eliminating one of these factors. Painting or enamelling a metal surface creates a buffer between the metal and the moisture in the atmosphere. Sacrificial coating is the method of coating a metal surface with another metal that is more likely to oxidize.
What is dry and wet corrosion?
When there is no water or moisture to help the corrosion, dry corrosion occurs, and the metal oxidizes on its own. Metals are corroded wet by electron transfer, which involves two processes: oxidation and reduction. The metal that loses electrons is known as the anode.
What metal is corrosion-resistant?
Corrosion-resistant materials such as copper and its alloys, brass, and bronze are examples of soft metals, or red metals. Copper is malleable, ductile, and a good heat and electricity conductor. These metals can provide corrosion resistance for a component’s entire life cycle.
Comments