Class 11 chemistry important questions with answers are provided here for Chapter 10 – The s-Block Elements. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 11 chemistry syllabus. By practising these Class 11 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 11 Annual examinations as well as other entrance exams such as NEET and JEE.
Download Class 11 Chemistry Chapter 10 – The s-Block Elements Important Questions with Answers PDF by clicking on the button below.
Recommended Video
S- Block Elements Class 11 Chemistry One Shot

Class 11 The s-Block Elements Important Questions with Answers
Short Answer Type Questions
Q1. How do you account for the strong reducing power of lithium in aqueous solution?
Answer:
Lithium’s strong reducing power in an aqueous solution can be explained in terms of electrode potential, which measures an element’s ability to lose electrons in an aqueous solution. It is mostly determined by the three criteria listed below. I.e.,
Li(s) → Li(g) ………. Sublimation enthalpy
Li(g) → Li+(g) + e– ……… Enthalpy of ionisation
Li+(g) + H2O → Li+(aq) ……… Enthalpy of hydration
Lithium has the highest hydration enthalpy due to its tiny size. However, Li has the highest ionisation enthalpy among alkali metals; and, hydration enthalpy takes precedence over ionisation enthalpy.
Because of its high enthalpy of hydration, lithium is the most powerful reducing agent in an aqueous solution.
Q2. When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li,
Na, and K.
Answer:
As the atomic size of an alkali metal decreases, its reactivity toward oxygen increases. Thus, Li produces just lithium oxide (Li2O), sodium produces mostly sodium peroxide (Na2O2) with a trace of sodium oxide, and potassium produces only potassium superoxide (KO2).
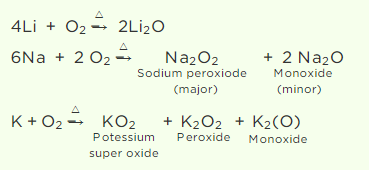
The superoxide ion, O2–, is only stable in the presence of massive cations like K, Rb, and others.
Q3. Complete the following reactions

Answer:
i) Peroxide ions react with water to form H2O2.
O22- + 2H2O → 2OH– + H2O2
ii) Superoxides react with water to form H2O2 and O2.
2O2– + 2H2O → 2OH– + H2O2 + O2
Q4. Lithium resembles magnesium in some of its properties. Mention two such properties and
give reasons for this resemblance.
Answer:
Lithium is similar to magnesium in that its charge size ratio is similar to magnesium. The association between it and Mg is known as a diagonal relationship.
The periodic qualities, in general, indicate an increasing or decreasing tendency throughout the group and vice versa along the period, bringing the diagonally located elements closer in value.
The following features should be noted:
- Li and Mg chlorides are deliquescent and soluble in alcohol and pyridine due to their covalent character.
- When Li and Mg carbonates are heated, they break down and release CO2.
Li2CO3 → Li2O + CO2
MgCO3 → MgO + CO2
Q5. Name an element from Group 2 which forms an amphoteric oxide and a water soluble sulphate.
Answer:
Beryllium is a group 2 element that generates an amphoteric oxide and a water-soluble sulphate.
Beryllium oxides have the formula BeO. In nature, all other alkaline earth metal oxides are basic. BeO is amphoteric, meaning it reacts with both acids and bases. Consider the amphoteric nature of Al2O3:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Beryllium sulphate is a white substance that crystallises as hydrated salts (BeSO4.4H2O).
Because it has the highest hydration energy of the group, BeSO4 is fairly soluble in water (small size). Because the hydration energy of BeSO4 is greater than the lattice energy, they are easily soluble.
Q6. Discuss the trend of the following:
- (i) Thermal stability of carbonates of Group 2 elements.
- (ii) The solubility and the nature of oxides of Group 2 elements.
Answer:
i) All alkaline earth metals (group 2) create carbonates with the general formula MCO3. Heating causes them to disintegrate into CO2 and metal oxides. The carbonates’ thermal stability improves as they progress through the group.
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3.
ii) Except for BeO, which is amphoteric and covalent, all oxides are basic and ionic. The lattice energy of oxides reduces as the size of the cation increases. The basic nature of the group increases as well. Except for BeO and MgO, all are water-soluble and release a lot of heat when they dissolve. The high lattice energy of BeO and MgO accounts for their insolubility.
Q7. Why are BeSO4 and MgSO4, readily soluble in water while CaSO4, SrSO4 and BaSO4, are
insoluble?
Answer:
Because of their high hydration enthalpies, BeSO4 and MgSO4 are readily soluble in water, but CaSO4, SrSO4, and BaSO4 are insoluble due to their low hydration enthalpies.
Because of the small size of the Be2+ and Mg2+ ions, the hydration enthalpies of BeSO4 and MgSO4 are very high, and they overcome the lattice enthalpy, making their sulphates soluble in water.
CaSO4, SrSO4, and BaSO4 have insufficient hydration enthalpies to overcome their lattice enthalpies, and as a result, they are water-insoluble.
Q8. All compounds of alkali metals are easily soluble in water but lithium compounds are more
soluble in organic solvents. Explain.
Answer:
The smallest size of the Li+ ion among all alkali metals and its high polarising power are the two factors that cause lithium compounds to develop covalent character (Fajan’s rule).
Other alkali metal compounds are ionic. As a result, they are water-soluble. Because lithium compounds are moderately covalent, they dissolve in alcohol and other organic solvents according to the principle of “like dissolves like.”
Q9. In the Solvay process, can we obtain sodium carbonate directly by treating the solution
containing (NH4)2CO3 with sodium chloride? Explain.
Answer:
No. We cannot obtain sodium carbonate directly by treating the (NH4)2CO3 solution with NaCl, sodium chloride. Carbon dioxide is passed through a concentrated sodium chloride solution saturated with ammonia in the Solvay process, resulting in ammonium carbonate and ammonium hydrogen carbonate. The crystals of ammonium hydrogen carbonate separate and are heated to generate sodium carbonate. The NH3 is extracted from the NH4Cl-containing solution, then heated and treated with Ca(OH)2. The reaction of (NH4)2CO3 with NaCl produces two products, Na2CO3 and NH4Cl, which are soluble in water; hence the equilibrium does not change to the right.
(NH4)2CO3 ⇌ NH4Cl + Na2CO3
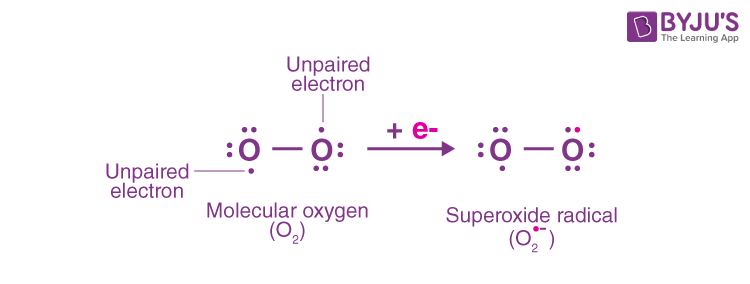
Q10. Write the Lewis structure of O2– ion and find out oxidation state of each oxygen atom? What is
the average oxidation state of oxygen in this ion?
Answer:
The oxidation state of an oxygen atom with no negative charge is 0 since it possesses 6 electrons. With a single negative charge, the oxygen atom contains 7 electrons. As a result, this oxygen atom’s oxidation state is -1. Thus, the average oxidation state = (-1 + 0)/2 = -1/2.
Q11. Why do beryllium and magnesium not impart colour to the flame in the flame test?
Answer:
The Bunsen flame takes on the colour of all alkaline earth metals (excluding Be and Mg). Distinct energies are necessary for electronic excitation and de-excitation, resulting in different colours.
Because of their small size, Be and Mg atoms link their electrons more tightly (because of higher effective nuclear charge). As a result, they demand a lot of excitation energy and do not excite the flame’s energy, resulting in no flame colour.
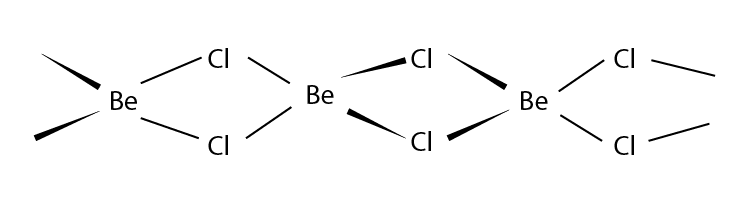
Q12. What is the structure of BeCl2 molecule in gaseous and solid state?
Answer:
In its gaseous state, beryllium chloride exists as a dimer (Be2Cl4), dissociating to the monomer at a temperature of roughly 1200K and has a linear shape. The structure of BeCl2 in the gaseous state is as follows:
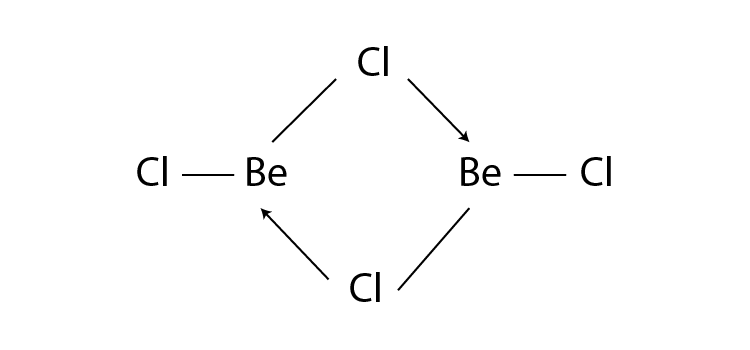
BeCl2 has a polymeric chain-like structure in the solid state. The Be atom is surrounded by four Cl atoms, two of which are covalently bonded, and the other two are coordinately bonded. The structure of BeCl2 in the solid-state is as follows:
Long Answer Type Questions
Q1. The s-block elements are characterised by their larger atomic sizes, lower ionisation
enthalpies, invariable +1 oxidation state and solubilities of their oxosalts. In the light of
these features describe the nature of their oxides, halides and oxosalts.
Answer:
Alkali metals rapidly form cation due to their low ionisation energy and large atomic size, and hence their compounds are ionic.
Oxides: Alkali metals produce normal oxides with the general formula M2O. When heated in air, only Li produces the typical oxide Li2O. Peroxide and superoxide are two further types of oxygen. Alkali metal oxides are very basic and soluble in water. Because of the increased ionic character, the basic character of oxide gradually increases from Li2O to Cs2O.
Halides: All alkali metal halides are ionic except lithium halides. Lithium halide is covalent due to the tremendous polarising power of Li+. Alkali metal halides have the generic formula MX due to their +1 oxidation states. Ionic halides can be formed because of the low ionisation enthalpy.
Oxosalts: Solid carbonates of the general formula M2CO3 are formed by all alkali metals. Carbonates are stable, except for Li2CO3, which is unstable and decomposes due to the strong polarising capacity of Li+. Except for Li, all alkali metals create solid bicarbonates, MHCO3. All alkali metals form nitrates with the formula MNO3. They are electrovalent chemicals that are colourless and water-soluble.
Q2. Present a comparative account of the alkali and alkaline earth metals with respect to the
following characteristics:
o (i) Tendency to form ionic / covalent compounds
o (li) Nature of oxides and their solubility in water
o (iii) Formation of oxosalts
o (iv) Solubility of oxosalts
o (v) Thermal stability of oxosalts
Answer:
Alkali metals:
i) All alkalis, except for Li, produce ionic compounds.
ii) The solubility of alkali metal oxides increases as one moves down the group.
iii) Oxosalts, such as carbonates, sulphates, and nitrates, are formed by all alkali metals.
iv) Carbonates and sulphates have increasing solubility as they progress through the group.
v) Heating causes Li carbonates and sulphates to disintegrate, but carbonates and sulphates of other metals become more stable as you progress down the group.
Alkaline earth metals:
i) All alkaline earth metals form ionic compounds except for Be.
ii) From Mg to Ba, the solubility of oxides of Mg, Ca, Sr, and Ba increases. BeO, on the other hand, is covalent and water-insoluble.
iii) Oxosalts, such as carbonates, sulphates, and nitrates, are formed by all alkaline earth metals.
iv) Carbonates and sulphates lose their solubility as they progress through the group.
v) Alkaline earth metal carbonates and sulphates all disintegrate when heated, but the “temperature of their decomposition increases along with the group, i.e., their thermal stability increases.”
Q3. When a metal of group 1 was dissolved in liquid ammonia, the following observations were
obtained:
(i) Blue solution was obtained initially.
(ii) On concentrating the solution, blue colour changed to bronze colour. How do you account for the blue colour of the solution? Give the name of the product
formed on keeping the solution for some time.
Answer:
i) When an alkali metal is dissolved in liquid ammonia, it undergoes a process that is,
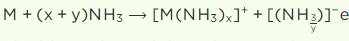
The solution’s blue colour comes from the existence of an ammoniated electron, which absorbs energy in the visible region of light and so gives it a blue hue.
ii) Due to metal ion clusters in concentrated solutions, the blue colour turns to bronze. After some time, the blue solution steadily releases hydrogen through the creation of amide.
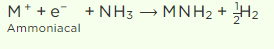
Q4. The stability of peroxide and superoxide of alkali metals increase as we go down the
group. Explain giving reason.
Answer:
As the size of the metal ion rises, the stability of peroxide or superoxide increases, i.e. KO2 < RbO2 < CsO2. Because of the strong positive charge around each alkali metal cation, alkali metals react with oxygen to generate various oxides. The smallest ion is Li+, which prevents the O2- ion from reacting with O2. Although Na+ has a greater positive field than Li, its positive field is weaker. It will not stop the conversion of O2- to O22-.
The largest ions, K+, Rb+, and Cs+, allow O22- to combine with O2 and generate the superoxide ion O2–.
Furthermore, increased peroxide or superoxide stability with increasing metal ion size is due to the stabilisation of large anions by larger cations via the lattice energy effect.
Q5. When water is added to compound (A) of calcium, solution of compound (B) is formed.
When carbon dioxide is passed into the solution, it turns milky due to the formation of
compound (C). If excess of carbon dioxide is passed into the solution milkiness disappears
due to the formation of compound (D). Identify the compounds A, B, C and D. Explain
why the milkiness disappears in the last step.
Answer:
The presence of milkiness in the solution of compound B after the addition of CO2 suggests that compound B is lime water, Ca(OH)2 and compound C is CaCO3. Because compound B is made by mixing H2O with compound A, compound A is quicklime, CaO.
The reactions are as follows:
CaO + H2O → Ca(OH)2
Ca(OH)2 + CO2 → CaCO3 + H2O.
Due to the synthesis of soluble calcium bicarbonate when an excess of CO2 is passed, milkiness vanishes (D).
CaCO3 + CO2 + H2O → Ca(HCO3)2
Q6. Lithium hydride can be used to prepare other useful hydrides. Beryllium hydride is one of them. Suggest a route for the preparation of beryllium hydride starting from lithium
hydride. Write chemical equations involved in the process.
Answer:
It is not possible to make BeH2 by heating powdered beryllium with dihydrogen. However, it can be made from the equivalent halides by reducing them with complicated alkali metal hydrides such as lithium aluminium hydride.
When beryllium hydride is made from lithium hydride, the following reaction takes place.
8LiH + Al2Cl6 → 2LiAlH4 + 6LiCl
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Q7. An element of group 2 forms covalent oxide which is amphoteric in nature and dissolves in
water to give an amphoteric hydroxide. Identify the element and write chemical reactions
of the hydroxide of the element with an alkali and an acid.
Answer:
Be is the only element in group 2 that produces the amphoteric covalent oxide BeO.
Ionic oxides, which are basic in nature, are formed by the remaining elements in this group. When BeO is dissolved in water, it produces a sparingly soluble hydroxide that interacts with acid and base to form salt.
BeO + H2O → Be(OH)2
Be(OH)2 + 2OH– → [Be(OH)4]2-
Beryllate ion:
Be(OH)2 + 2HCl + 2H2O → [Be(OH)4]Cl2
Q8. Ions of an element of group 1 participate in the transmission of nerve signals and
transport of sugars and amino acids into cells. This element imparts yellow colour to the
flame in a flame test and forms an oxide and a peroxide with oxygen. Identify the element
and write a chemical reaction to show the formation of its peroxide. Why does the element
impart colour to the flame?
Answer:
The presence of a yellow flame in the flame test suggests that the alkali metal is sodium. It combines with oxygen to generate sodium peroxide Na2O2 and sodium oxide Na2O.
4Na + O2 → 2Na2O
2Na + O2 → Na2O2
2Na2O + O2 → 2Na2O2
The enthalpy of ionisation of sodium is low. When sodium metal or its salt is burned in a Bunsen flame, the flame energy excites the outermost electron, which then returns to its original location and emits the absorbed energy as visible light, with the complementary colour of the absorbed colour visible. That’s why the flame becomes yellow when sodium is present.

Comments