Class 11 chemistry important questions with answers are provided here for Chapter 9 Hydrogen. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 11 chemistry syllabus. By practising these Class 11 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 11 Annual examinations as well as other entrance exams such as NEET and JEE.
Download Class 11 Chemistry Chapter 9 Hydrogen Important Questions with Answers PDF by clicking on the button below.
Recommended Video
Hydrogen Class 11 Chemistry One Shot

Class 11 Hydrogen Important Questions with Answers
Short Answer Type Questions
Q1. How can production of hydrogen from water gas be increased by using a water gas shift reaction?
Answer:
The water-gas shift reaction is a chemical reaction in which carbon monoxide interacts with steam to produce carbon dioxide and pure hydrogen in the presence of a catalyst.
(CO(g) + H2(g)) the mixture is a water gas.
The following is the reaction for producing pure hydrogen from water gas:
CO + H2 + H2O → CO2 + 2H2
Q2. What are metallic/interstitial hydrides? How do they differ from molecular hydrides?
Answer:
A binary compound having an element and a hydrogen atom is known as a hydride.
Interstitial hydrides are another name for metallic hydrides. Metallic/interstitial hydrides are compounds that involve the bonding of transition metals and hydrogen. Many d-Block and f-Block elements come from them. These hydrides are heat and electricity conductors.
The hydrides in which more electronegative atoms link with the H-atom are known as molecular hydrides. Metallic hydrides have high electrical conductivity, but molecular hydrides have a low conductivity. Metallic hydrides exist in a solid-state, whereas molecular hydrides exist in a gaseous state.
The bonding of hydrogen and transition metals forms interstitial or metallic hydrides, which differ from molecular hydrides in that molecular hydrides contain bonding between an electronegative atom and hydrogen.
Q3. Name the classes of hydrides to which H2O, B2H6, and NaH belong.
Answer:
H2O belongs to the electron-rich covalent hydride/molecular hydride or covalent hydride class.
B2H6 belongs to electron-deficient molecular hydride or covalent hydride class.
NaH belongs to the ionic hydride class.
Q4. If the same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Answer:
Density is defined as the mass per unit volume (i.e. mass/volume). Because water expands as it freezes, the amount of ice for the same quantity of water is more than the volume of liquid water. In other words, because ice has a lower density than liquid water, it floats on water.
Q5. Complete the following equations:

Answer:
(i) PbS + 4H2O2 → PbSO4 + 4H2O [Redox reaction]
(ii) CO + 2H2 → CH3OH [Redox reaction]
Q6. Give reasons:
(i) Lakes freeze from top towards bottom.
(ii) Ice floats on water.
Answer:
(i) Because the temperature drops over the winter, the lake freezes from top to bottom, and the movement of water is such that the cold water is heavier and sinks to the bottom. Warm water replaces it as it rises to the surface. The cycle continues until the temperature drops below 4 degrees and the lake freezes from top to bottom.
(ii) Ice has a lower density than water due to its structure, which creates empty spaces between water molecules (four hydrogen atoms surround one oxygen atom), allowing it to float on water.
Q7. What do you understand by the term ‘auto protolysis of water’? What is its significance?
Answer:
Autoprotolysis of water is the reaction that produces hydronium ion and hydroxide ion from two water molecules. Water self-ionises in this way.
2H2O → H3O+ + OH–
The amphoteric characteristic of water is demonstrated by this reaction. It can function as both an acid and a base. One water molecule provides an electron, while the other absorbs an electron.
Q8. Discuss briefly de-mineralisation of water by ion exchange resin.
Answer:
Water demineralisation refers to the removal of all soluble salts in the water via cation and anion exchange. Sodium, calcium, and magnesium cations replace hydrogen cations in the cation exchange process. OH is exchanged during the anion exchange process. Both of these factors interact to generate water.
H+ +OH– → H2O
Q9. Molecular hydrides are classified as electron deficient, electron precise and electron rich compounds. Explain each type with two examples.
Answer:
Electron deficient: An electron-deficient compound is one in which the number of electrons required to complete the octet of the central atom is insufficient. There aren’t enough electrons in these compounds to make typical electron-pair bonds between each pair of linked atoms.
Examples: Electron-deficient compounds, such as B2F6, Al2Cl6, and others, have less than 8 electrons in their valence shells. Electron excess compounds, on the other hand, are those with more than 8 electrons in the valence shells, such as SF6, O8F8, and so on.
Electron precise: Hydrogen compounds that are electron precise contain enough valence electrons to form covalent bonds. Electron precise hydrides are those that have the correct quantity of electrons needed to establish a covalent connection. Group 14 elements are commonly used to create these compounds. The compounds are typically tetrahedral in form.
Examples: CH4, SiH4, etc.
Electron rich: Electron-rich hydrides are hydrides that have more electrons than are required for bonding. The lone pair of electrons on the core atom account for the majority of the extra electrons. These compounds are mainly made up of elements from groups 15, 16, and 17.
Examples: NH3, PH3, etc.
Q10. How is heavy water prepared? Compare its physical properties with those of ordinary water.
Answer:
Extensive electrolysis of water can be used to make heavy water.
Comparison of physical properties of H2O and D2O is as follows:
| S.R.No. | Property | H2O | D2O |
|---|---|---|---|
| (i) | Molecular mass (g/mol) | 18.015 | 20.027 |
| (ii) | Density(298)g/cm | 1.0000 | 1.1059 |
| (iii) | Boiling point (K) | 373.0 | 374.4 |
| (iv) | Melting point (K) | 273.0 | 276.8 |
| (v) | Enthalpy of vaporisation (kJ/mol) | 40.6 | 41.61 |
Q11. Write one chemical reaction for the preparation of D2O2.
Answer:
The reaction of D2SO4 dissolved in water over BaO2 produces D2O2.
BaO2 + D2SO4 → BaSO4 + D2O
Q12. Calculate the strength of 5 volume H₂O₂ solution.
Answer:
2H2O2(l) → 2H2O(l) + O2(g)
At standard temperature and pressure (STP), one litre of H2O2 produces five litres of O2.
It is obvious from the equation that 68g of H2O2 produces 22.4L of O2.
Thus, 5L of O2 is produced by = (68g×5L)/22.7L = 3400/224g of H2O2 = 15.18g of H2O2
∴ When 15.18g of H2O2 is dissolved in 1L of water, it produces 5L of oxygen, or 15.18g H2O2/1000mL solution. As a result, an H2O2 solution with a concentration of 15.18 g/L (1.518%) is known as a 5V solution.
Q13. (i) Draw the gas phase and solid phase structure of H₂O₂.
(ii) H₂O₂ is a better oxidising agent than water. Explain.
Answer:
(i) The gas and solid phases of H2O2 have slightly different structures.
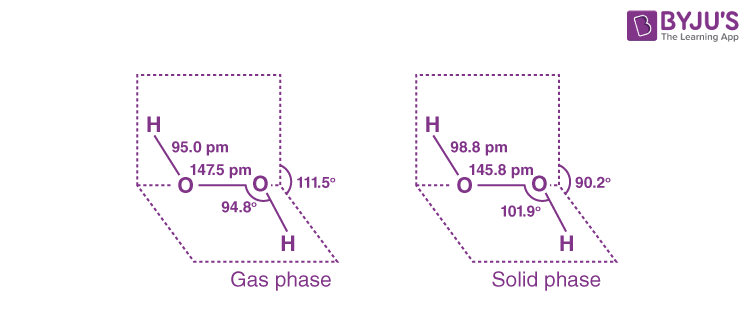
(ii) H₂O₂ is a better oxidising agent than water because it converts an acidified KI solution to I2, resulting in blue colour in the starch solution but not in water. In addition, water does not convert black PbS to white PbSO4, but H2O2 does.
14. Melting point, enthalpy of vapourisation and viscosity data of H₂O and D₂O is given below :
| H2O | D2O | |
|---|---|---|
| Melting point / K | 373.0 | 374.4 |
| Enthalpy of vapourisation at (373 K)/ kJ mol¹ | 40.66 | 41.61 |
| Viscosity/centipoise | 0.8903 | 1.107 |
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
Answer:
All of these products’ melting points, enthalpy of vapourisation, and viscosity values are determined by intermolecular forces of attraction. Intermolecular forces of attraction are stronger in D2O than in H2O because their values are higher in D2O than in H2O.
Q15. Dihydrogen reacts with dioxygen (O₂) to form water. Write the name and formula of the product when the isotope of hydrogen which has one proton and one neutron in its nucleus is treated with oxygen. Will the reactivity of both the isotopes be the same towards oxygen? Justify your answer.
Answer:
Deuterium (D) is a hydrogen isotope that has one proton and one neutron. Deuterium oxide, or heavy water, is generated when dideuterium combines with dioxygen.
H2 and D2 will have distinct reactions with oxygen. Because the D–D bond is stronger than the H–H bond, H2 is more reactive than D2 when it comes to oxygen reactions.
Q16. Explain why HCI is a gas and HF is a liquid.
Answer:
Intermolecular hydrogen bonding binds HF molecules together. As a result, at room temperature, HF is a liquid. It exists as a gas at room temperature since intermolecular hydrogen bonding is not present in the HCl molecules.
Q17. When the first element of the periodic table is treated with dioxygen, it gives a compound whose solid-state floats on its liquid state. This compound has an ability to act as an acid as well as a base. What products will be formed when this compound undergoes autoionisation?
Answer:
H is the first element in the periodic table, and dihydrogen is its molecular form (H2). Water is created when dihydrogen interacts with dioxygen. At room temperature, water is a liquid. Liquid water expands to make ice as it freezes. In other words, because ice has a lesser density than liquid water, it floats on top of it.
In nature, water is amphoteric, meaning it functions as an acid in the presence of strong bases and as a base in the presence of strong acids.
H2O(l) + NH3(aq) → NH4+(aq) + OH–(aq)
H2O(l) + H2S(aq) → H2O+(aq) + HS–(aq)
Water undergoes self-ionisation as a result of its amphoteric nature, as seen below:
H2O(l) + H2O(base) → H3O+(aq) + OH–(aq)
Formation of conjugate acid and conjugate base takes place. Auto-protolysis is the term for this self-ionisation of water.
Q18. Rohan heard that instructions were given to the laboratory attendant to store a particular chemical i.e., keep it in the dark room, add some urea in it, and keep it away from dust. This chemical acts as an oxidising as well as a reducing agent in both acidic and alkaline media. This chemical is important for use in the pollution control treatment of domestic and industrial effluents.
(i) Write the name of this compound.
(ii) Explain why such precautions are taken for storing this chemical.
Answer:
(i) The name of the compound is Hydrogen peroxide, H2O2, which in both acidic and basic media, acts as an oxidising and reducing agent.
(ii) The compound is made up of light and dust particles and is utilised in a variety of sectors, including textiles and paper. It is often used as a bleaching agent. It is stored in the dark because it can act as both an oxidising and reducing agent, and it is maintained away from dust since dust can cause explosive disintegration of the compound stabiliser.
Q19. Give reasons why hydrogen resembles alkali metals?
Answer:
In the following ways, hydrogen resembles alkali metals from group I of the periodic table, such as Li, Na, K, Rb, and Cs.
i) Hydrogen, like alkali metals, has one electron in its outermost (valence) shell and has an oxidation state of +1.
ii) Hydrogen, like alkali metals, loses its single electron to produce hydrogen ion, or H+ (proton).
iii) Hydrogen, like alkali metals, reacts with electronegative non-metals like oxygen, halogens, and sulphur to generate oxides, halides, and sulphides, respectively.
iv) Hydrogen, like alkali metals, is a powerful reducing agent.
Q20. Hydrogen generally forms covalent compounds. Give reason.
Answer:
Because hydrogen shares its electron, it tends to create covalent compounds. Hydrogen is a single-electron element with an atomic number of one. It prefers to share electrons with other atoms since it cannot lose an electron and hence creates covalent compounds.
Q21. Why is the Ionisation enthalpy of hydrogen higher than that of sodium?
Answer:
Because of the stronger nuclear attraction, removing a valence electron from the H atom requires more energy than removing one from the Na atom. As a result, ionisation enthalpy of hydrogen (1312 kJ/mol) is larger than sodium (496 kL/mol).
Q22. Basic principle of hydrogen economy is transportation and storage of energy in the form of liquid or gaseous hydrogen. Which property of hydrogen may be useful for this purpose? Support your answer with the chemical equation if required.
Answer:
At room temperature, hydrogen is a gas. Because of its mass, it is difficult to move it either by train or by road. However, gaseous H2 may be transformed to liquid H2 by cooling and applying high pressure, which has a much lower volume and can be transported more readily. Thus, one of the most important properties of hydrogen for hydrogen economy is that it can be turned into a liquid by cooling it under high pressure.
Q23. What is the importance of heavy water?
Answer:
Heavy water is employed as a neutron moderator in nuclear reactors, slowing down neutrons so that they are more likely to react with fissile uranium-235 rather than uranium-238, which collects neutrons without fissioning.
It is utilised as a tracer compound in the research of reaction mechanisms and for the synthesis of other deuterium compounds such as CD4, D2SO4 etc.
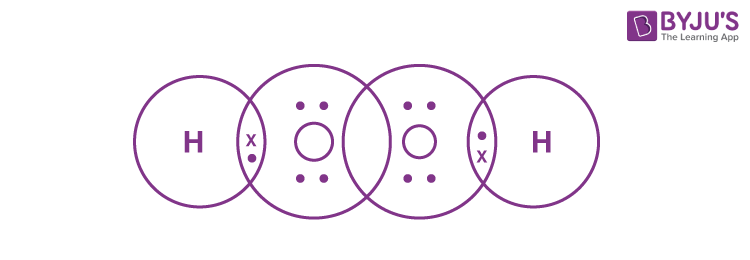
Q24. Write the Lewis structure of hydrogen peroxide.
Answer:
The Lewis structure of hydrogen peroxide is:
Q25. An acidic solution of hydrogen peroxide behaves as an oxidising as well as a reducing agent. Illustrate it with the help of a chemical equation.
Answer:
The chemical equation for H2O2, which acts as both an oxidising and reducing agent, is shown below:
i) Acidified KI is oxidised by H2O2 to iodine.
2KI + H2O2 + H2SO4 → I2 + K2SO4 → 2H2O
ii) Reactions that justify the reduction of nature include:
H2O2 + Cl2 → 2HCl + O2
Q26. With the help of suitable examples, explain the property of H₂O₂ that is responsible for its bleaching action?
Answer:
The bleaching effect of H2O2 is due to the labile oxygen it releases when it decomposes. H2O2 → H2O + [O] Coloured substance becomes colourless when nascent oxygen mixes with it. It can be used to bleach feathers, silk, wool, ivory, and other materials. Paper, oils, and fats can all be bleached with it.
Q27. Why is water molecule polar?
Answer:
The twisted structure of the water molecule (H2O) makes it polar. It’s a polar solvent as well. It has a positive and negative charge on one side. Two hydrogen atoms and one oxygen atom make up this molecule. The polar forces act to draw two water molecules together when they reach close enough. A water molecule’s oxygen atom will form a link with numerous hydrogen atoms from other water molecules.
Q28. Why does the water show a high boiling point as compared to hydrogen sulphide? Give reasons for your answer.
Answer:
Water experiences substantial H-bonding as a result of the high electronegativity of oxygen (E.N.=3.5), resulting in water existing as an associated molecule. The presence of hydrogen bonding between O and H in water molecules gives it a greater boiling point than hydrogen sulphide. The high boiling point is due to the strong strength of the hydrogen bond.
Sulphur, on the other hand, is less electronegative (E.N.=2.5), as hydrogen sulphide does not form hydrogen bonds and is a gas at ambient temperature. Molecules are free to migrate here, indicating that they are loosely packed.
Q29. Why can dilute solutions of hydrogen peroxide not be concentrated by heating? How can a concentrated solution of hydrogen peroxide be obtained?
Answer:
Heat cannot be used to concentrate H2O2 solution above 30% concentration since explosions are always a possibility. At 35-40°C, the solution is concentrated at a lowered pressure of around 15 mm. After several distillations, 90% H2O2 is obtained. Cooling with solid CO2 and an ether bath until crystallisation occurs allows for even more concentration. To obtain 99% pure H2O2, crystals are separated, melted, and then refrozen.
Q30. Why is hydrogen peroxide stored in wax lined bottles?
Answer:
The rough surfaces of glass, alkali oxides present in it, and light breakdown hydrogen peroxide. H2O2 is typically stored in coloured paraffin wax-coated plastic or Teflon bottles to prevent decomposition.
Q31. Why does hard water not form lather with soap?
Answer:
Bicarbonates, chlorides, and sulphates of Ca2+ and Mg2+ are all found in hard water. These react with soap molecules to create calcium and magnesium salts of soap precipitates. As a result, soap is squandered, and hard water does not form soap lather.
2C17H35COONa(aq) + M2+(aq) → (C17H35COO)2↓ + 2Na+(aq)
It is thus, not suitable for laundry.
Q32. Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Answer:
In the breakdown of hydrogen peroxide, sulphuric acid functions as a catalyst. As a result, mild acids such as phosphoric acid are utilised.
BaO2 + H2SO4 → BaSO4 + H2O2
The hydrogen peroxide generated is further decomposed by the sulphuric acid.
2H2O2 → O2 + 2H2O
The resultant yield will be lower. As a result, moderate acids such as phosphoric acid H3PO4, H3CO3, and others are utilised.
The reaction will be as follows:
3BaO2 + 2H3PO4 → Ba3(PO4)2↓ + 3H2O2
The Ba3(PO4)2 crystallises into an insoluble precipitate.
Q33. How will you account for 104.5° bond angle in water?
Answer:
Two of an oxygen atom’s six electrons are bound to a hydrogen atom, leaving two lone pairs of electrons. The bond angle in H2O is 104.5° due to the presence of these lone pairs of electrons. The valence shell electron pair repulsion hypothesis can be used to explain this (VSEPR).
The oxygen in the H2O molecule is sp3 hybridised, resulting in a tetrahedral structure. H atoms occupy two locations by creating sigma bonds with two hybrid orbitals, and lone pairs occupy two positions. The projected bond angle is 109.5°, but the actual bond angle is 104.5°. The repulsions between lone pairs are larger than the repulsions between bond pairs. As a result, the bending angle in water drops from 109.5 to 104.5°.
Q34. Write redox reactions between fluorine and water.
Answer:
F2 is an extremely powerful oxidiser. It decomposes H2O into O2 or O3.
2F2(g) + 2H2O(l) →O2(g) + 4H+(aq) + 4F−(aq)
3F2(g) + 3H2O(l) → O3(g) + 6H+(aq) + 6F−(aq)
Q35. Write two reactions to explain the amphoteric nature of water.
Answer:
Water has the ability to act as both a base and an acid, making it an amphoteric material. According to the Bronsted lowry theory, it functions as an acid with NH3 and a base with H2S.
H2O(l) + NH3(aq) → OH−(aq) + 4F−(aq)
H2O(l) + H2S(aq) → H3O+(aq) + HS−(aq)
Water undergoes auto-protolysis (self-ionisation) and the reaction is as follows:
H2O(l) + H2O(l) → H3O+(aq) + OH−(aq)
Formation of conjugate acid and conjugate base takes place.
Long Answer Type Questions
Q1. Atomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
Answer:
Hydrogen atoms are extremely unstable. Because atomic hydrogen’s electronic configuration is 1s1, it requires one more electron to complete its configuration and achieve stability. As a result, atomic hydrogen is extremely reactive, combining with practically every element. It, on the other hand, reacts in three separate ways, namely,
i) due to the loss of a single electron to H+
ii) by gain of one electron to from H− and
iii) by forming single covalent connections by sharing its electron with other atoms. The bond dissociation energy of the H-H bond, on the other hand, is extremely high (435.88 kJ/mol1). As a result, molecular hydrogen is nearly inert at ambient temperature, reacting with only a few elements.
Q2. How can D2O be prepared from water? Mention the physical properties in which D₂O differs from H2O. Give at least three reactions of D2O showing the exchange of hydrogen with deuterium.
Answer:
D2O is produced by prolonged electrolysis of water; it varies from water in that it has a large molecular mass.
| H2O | D2O | |
|---|---|---|
| Melting point | 273K | 276.8K |
| Boiling Point | 373K | 373.4K |
| Molecular Mass | 18.016 | 20.3 |
Below reactions are showing the exchange of Hydrogen with deuterium:
- NaOH + D2O → NaOD + HOD
- HCl + D2O → DCl + HOD
- NH4Cl + D2O → NH3DCl + HOD
Q3. How will you concentrate H₂O₂? Show differences between structures of H₂O₂ and H₂O by drawing their spatial structures. Also mention three important uses of H₂O₂.
Answer:
We can employ evaporation and barium peroxide to make hydrogen peroxide by removing extra water. We utilise distillation and low pressure to make it more concentrated, and then pure hydrogen peroxide can be obtained.
The spatial structures of H2O and H2O2 are given below: –
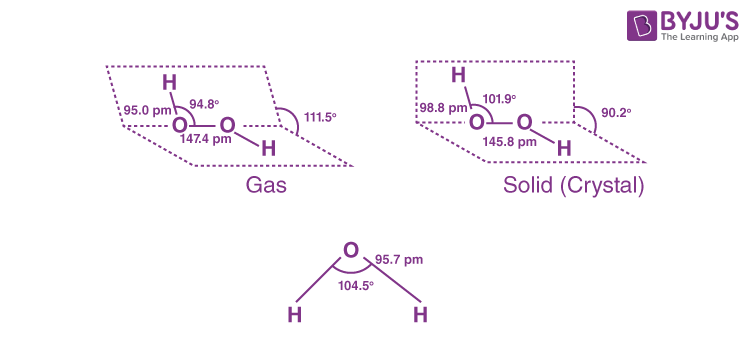
Three important uses of H2O2 are:
i) In the market, peroxide is used as a disinfectant and antiseptic.
ii) It’s utilised in the industry to make other compounds, and it’s also employed as a commercial bleaching agent.
iii) In addition, it has a lot of uses in the textile industry.
Q4. (i) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
(ii) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations.
Answer:
i) The auto-oxidation of two alkylanthraquinols is used to produce H2O2.
H2O2 is generated at a rate of 1% in this situation. It is extracted with water and distilled under decreased pressure to a concentration of 30 percent (by mass). Careful distillation under low pressure can increase the concentration to 85%. To obtain pure H2O2, the remaining water can be frozen off.
ii) Oxidising effect in acidic medium is as follows:
2Fe2+(aq) + 2H+(aq) + H2O2(aq) + H2O2(aq) → 2Fe3+(aq) + 2H2O(l)
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O(l)
Reducing effect in acidic medium is as follows:
2MnO4− + 6H+ +5H2O2 → 2Mn2+(aq) + 8H2O + 5O2
HOCl + H2O2 → H3O+ +Cl− + O2
Oxidising effect in basic medium is as follows:
2Fe2+ + H2O2 → 2Fe3+ + 2OH−
Mn2+ + H2O2 → Mn4+ + 2OH−
Reducing effect in basic medium is as follows:
I2 + H2O2 + 2OH− → 2I− + 2H2O + O2
2MnO−4 + 3H2O2 → 2MnO2 + 3O2 + 2H2O + 2OH−
Q5. What mass of hydrogen peroxide will be present in 2 litres of a 5 molar solution? Calculate the mass of oxygen which will be liberated by the decomposition of 200 mL of this solution.
Answer:
We know that the molecular mass of H2O2 = 34
Mass of H2O2 present in 1 molar solution = 34 g
∴ The mass of H2O2 present in 2 litres of 1 molar solution = 2 ×34 = 68g
Thus, the mass of H2O2 that is present in 200ml solution of 1 molar H2O2 would be
= 34/5 = 6.8g
2H2O2 → 2H2O + O2
Decomposition of 68g of H2O2 yields O2 = 32g.
∴ 34g of H2O2 on decomposition will give O2 = (32/68) × 34 = 16g.
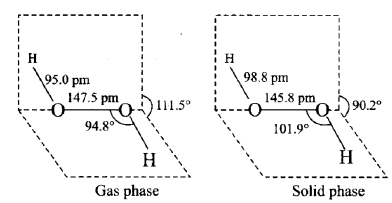
Q6. A colourless liquid ‘A’ contains H and O elements only. It decomposes slowly on exposure to light. It is stabilised by mixing urea to store in the presence of light.
(i) Suggest possible structure of A.
(ii) Write chemical equations for its decomposition reaction in light.
Answer:
i) A is Hydrogen Peroxide (H2O2), which decomposes slowly when exposed to light and can be stored in the presence of light by combining urea.
ii) The chemical equations for its decomposition reaction in light is:
2H2O2(l) → 2H2O(l) + O2(g)
Q7. An ionic hydride of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al2Cl6.
Answer:
It is LiH because the ionic hydride of an alkali metal has a considerable covalent character. LiH is almost unreactive towards O2 and Cl2 due to its high stability. It forms lithium aluminium hydride when it interacts with Al2Cl6.
8LiH + Al2Cl6 → 2LiAlH4 + 6LiCl
Q8. Sodium forms a crystalline ionic solid with dihydrogen. The solid is nonvolatile and non conducting in nature. It reacts violently with water to produce dihydrogen gas. Write the formula of this compound and its reaction with water. What will happen on electrolysis of the melt of this solid.
Answer:
Sodium hydride is a crystalline ionic solid formed when sodium interacts with dihydrogen.
2Na + H2 → 2Na + H−
It produces H2 gas when it interacts with H2O.
2NaH + 2H2O → 2NaOH + 2H2
Although the solid state of Na+ H– does not carry electricity, the electrolysis of its melt produces H2 at the anode and Na at the cathode.
Na+H– (l) → 2Na(l) + H2(g)
During electrolysis, dihydrogen gas is liberated at the anode, confirming the presence of H– ion.

Good and it`s so inspiration to meet the academic goals