Many of the reactions of sodium metal are surface-area dependent because it is solid. It works best as a reagent when cut into fine strips or wires rather than chunks. As you might expect, a reagent that reacts violently with water has the potential to be extremely dangerous. When used to remove traces of water from solvents in distillation setups, sodium is dangerous: chunks can be passivated with a surface layer of NaOH, and scratching the surface of the material during quench will expose reactive sodium metal, which can then react rapidly with the quenching agent, resulting in explosions and fires.
Table of Contents
- Sodium and Ammonia Reaction
- Ammonia and sodium reaction properties
- Sodium Metal in NH3 – Alkynes Reduction to Trans-Alkenes
- Sodium Metal in Ammonia: The Birch Reduction
- The Mechanism of Sodium in NH3 for Alkyne Reduction
- Frequently Asked Questions – FAQs
Sodium and Ammonia Reaction
Sodium reacts with ammonia gas to form sodamide and hydrogen gas. In this reaction, liquefied ammonia is typically used to prepare sodamide. The chemical equation for preparing sodamide is given below.
2Na + 2NH3 → 2NaNH2 + H2
This is an example of a redox reaction. Sodamide is also referred to as sodium amide and sodium azanide.
Ammonia and sodium reaction properties
- Fires may occur as a result of the formation of hydrogen gas.
- Ammonia gas can act as an acid – Normally, metals emit hydrogen gas when they react with acids such as sodium and dilute HCl. Since sodium is a metal, and hydrogen gas is produced as a byproduct, this reaction is similar to the metal-acid reaction. As a result, ammonia should have acidic properties as well.
- Ammonia is an oxidising agent (sodium is oxidized)- Ammonia is an oxidising agent because sodium is oxidised.
About sodium amide-
i. Sodium amide is a powerful base.
ii. Ammonia and sodium hydroxide are produced when sodium amide reacts violently with water.
Sodium Metal in NH3 – Alkynes Reduction to Trans-Alkenes
Sodium dissolves in liquid ammonia (boiling point –33 °C), resulting in a deep blue colour. When alkynes are present, they have reduced to the trans (i.e. E) alkene. As a result, Na/NH3 is an excellent companion to the Lindlar reduction of alkynes, which yields cis-alkenes.
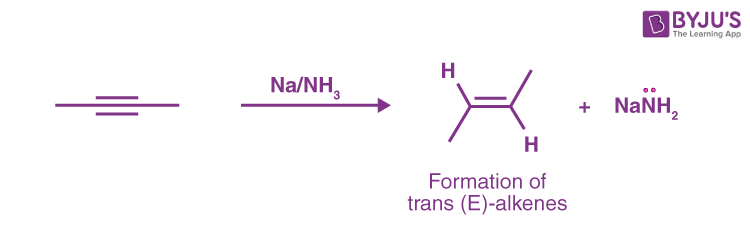
Sodium Metal in Ammonia: The Birch Reduction
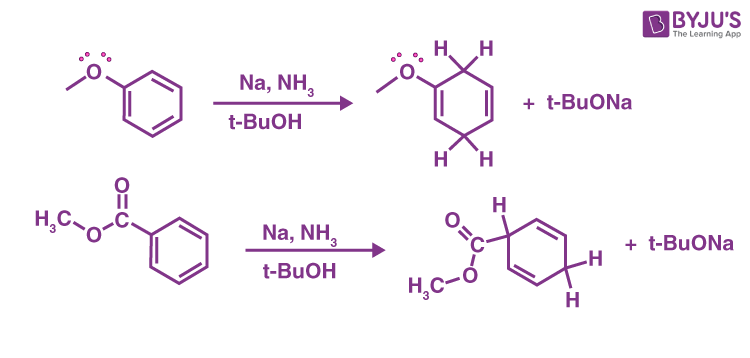
Aromatic compounds are reduced to (non-conjugated) dienes by sodium metal in ammonia (NH3) containing several equivalents of t-butanol. The Birch reduction is the name given to this useful reaction.
The pattern is determined by whether the aromatic group’s substituent is electron-donating (such as OCH3) or electron-withdrawing (such as CH3) (CO2Me).
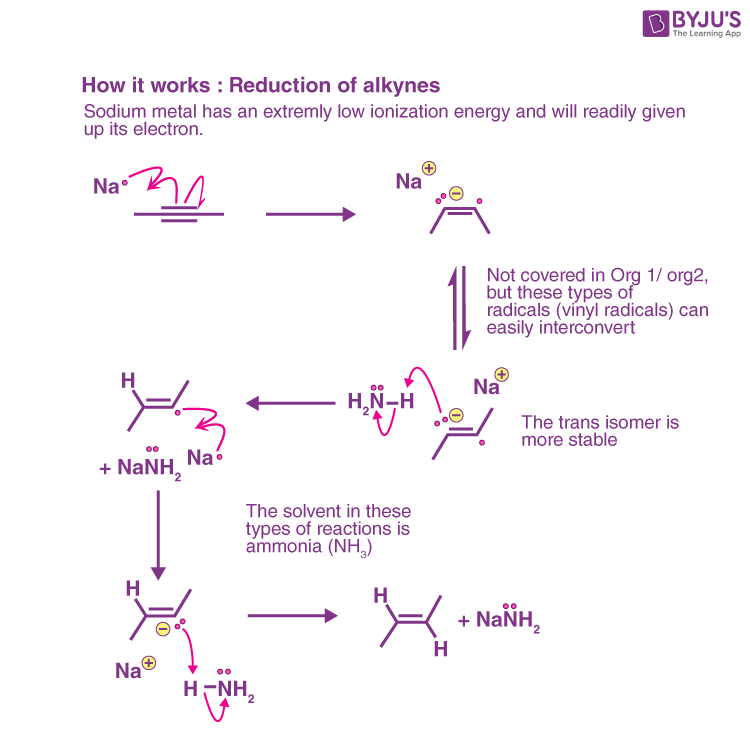
The Mechanism of Sodium in NH3 for Alkyne Reduction
When an alkyne is reduced by sodium, the C-C double bond is broken, and an anion adjacent to a radical is formed. The formed radical can interconvert between its cis and trans forms, but the trans form is generally more stable due to steric factors. The anion is then protonated by NH3 (the only acid in solution) to produce the vinyl radical, which is then reduced by the second equivalent of Na to produce a second anion. This is then protonated with a second equivalent of NH3 to yield the alkene. As a result, the net process yields a trans-alkene and two NaNH2 equivalents.
Frequently Asked Questions on Na NH3 Reaction
What substance is formed when Na reacts with liquid NH3?
When sodium reacts with ammonia, sodium amide, or NaNH2, is formed, and hydrogen gas is released.
How is alkyne converted to alkenes?
With the help of sodium dissolved in an ammonia solvent, alkynes can be converted to trans-alkenes. An electron is donated by a Na radical to one of the P bonds in a carbon-carbon triple bond. This results in forming an anion, which hydrogen can protonate in an ammonia solvent.
Why is a Na solution in liquid NH3 highly reactive?
Sodium ionises in liquid ammonia, producing a Na+ ion and an electron. Ammonia is used to dissolve the electron. This solvated electron (also known as an ammoniated electron) imparts reducing properties to the sodium solution in liquid ammonia.
What effect does Na NH3 have on benzene?
When benzene reacts with Na/Nh3, it produces 1,4-cyclohexadien.
What exactly is the Birch reduction mechanism?
The Birch reduction is an organic chemical reaction in which aromatic compounds with benzenoid rings are converted into 1,4-cyclohexadiene, a molecule with two hydrogen atoms attached at opposite ends. In synthetic organic chemistry, it is a very useful reaction.
Comments