The matter is anything that occupies space. Air is a matter which is inevitable in our lives. The earth is covered with a blanket of air called the atmosphere. Among the few fundamental elements, it is the most important because no life can exist for a pulse of time without it. It is needed by every single organism for their survival. In this session, let us understand the composition of air.
| Table of Contents: |
In addition to breathing, air has an influential role in abiotic components of the environment like wind, rain, climate, etc. Watch the video below to learn about the composition of air and properties in detail.

Chemical Composition of Air
Air is a mixture of gases which makes up the Earth’s atmosphere. These gases are colorless and odorless and hence, we can’t see them but only feel them. The atmosphere is an ocean of these gases. It consists of 78% nitrogen, 21% oxygen and 1 % other gases and water vapour. The composition of air does not change as you travel through the layers of the atmosphere. What changes is the number of molecules. The air molecules decrease and become less. The moisture content varies from place to place. Arid regions have less moisture content as compared to wetlands.
- The water vapour or moisture content of air varies. The maximum moisture carrying capacity of air depends primarily on temperature.
- The composition of air is unchanged until the elevation of approximately 10,000 m.
- The average air temperature diminishes at the rate of 0.6oC for each 100 m vertical height.
- “One Standard Atmosphere” is defined as the pressure which is equal to that exerted by a 760 mm column of mercury at 0°C sea level and at standard gravity (32.174 ft/sec2).
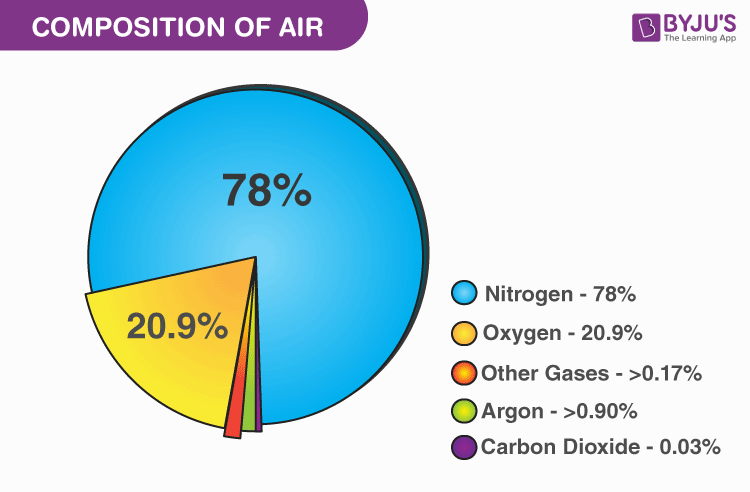
| Composition of Air | |||||
| Element | Volume by % | Weight by % | PPM(Parts per Million) by Volume | Symbol of the Element | Molecular Weight of the element |
| Nitrogen | 78.08 | 75.47 | 780790 | N2 | 28.01 |
| Oxygen | 20.95 | 23.20 | 209445 | O2 | 32.00 |
| Argon | 0.93 | 1.28 | 9339 | Ar | 39.95 |
| Carbon Dioxide | 0.040 | 0.062 | 404 | CO2 | 44.01 |
| Neon | 0.0018 | 0.0012 | 18.21 | Ne | 20.18 |
| Helium | 0.0005 | 0.00007 | 5.24 | He | 4.00 |
| Krypton | 0.0001 | 0.0003 | 1.14 | Kr | 83.80 |
| Hydrogen | 0.00005 | Negligible | 0.50 | H2 | 2.02 |
| Xenon | 8.7 x 10-6 | 0.00004 | 0.087 | Xe | 131.30 |
Other Components of Air
Some other components of air are mentioned below:
- Sulfur dioxide(SO2) – 1.0 ppm
- Methane(CH4 )-2.0 ppm
- Nitrous oxide(N2O) – 0.5 ppm
- Ozone(O3)-0 to 0.07 ppm
- Nitrogen dioxide(NO2) – 0.02 ppm
- Iodine(I2)-0.01 ppm
- Carbon monoxide(CO) – 0 to trace ppm
- Ammonia(NH3)-0 to trace ppm
Hope you have understood the composition of air in detail with the help of the chart given above. Let us know the properties of air.
Properties of Air
As mentioned earlier, gases are matter. There are certain properties of gases like any other matter. Some common properties are as follows:
- Colourless and Odourless:
Air generally has no colour or odour. It is an invisible matter that can only be felt. All living things breathe air for their survival. Moving air is called wind.
- Occupy Space:
It is a mixture of different gases. Hence, like every other matter, they also occupy space. On blowing, a balloon expands because the air being blown into it fills up the empty space.
- Air Exerts Pressure:
It has weight, and the pressure exerted by the weight of air is known as air pressure. Due to gravity, this mixture of gases near the surface is denser than at high altitudes. This is why the gaseous atmosphere in the mountains is thinner than that at the surface.
- Expansion:
Another property is its expanding property. On heating, it expands and occupies more space. The more it expands, the thinner it becomes. Hence, the pressure of the warm wind is lower than that of cold wind.
Frequently Asked Questions – FAQs
Does air occupy space?
What are the properties of air?
The following are the properties of air:
- Colourless
- Odourless
- Air exerts pressure and occupies space
- Air undergoes expansion
List the major components of air.
Major components of air are:
- Nitrogen
- Oxygen
- Argon
- Carbon di-oxide
- Neon and other gases
What is meant by atmosphere?
Name the different layers of Earth’s atmosphere?
Layers of Earth’s atmosphere are:
- Exosphere
- Thermosphere
- Mesosphere
- Stratosphere
- Troposphere
After learning the composition of air and properties of air, now learn about layers of the earth. Stay tuned with BYJU’S for more such interesting articles.


Thank you , BYJU’S is the best way to learn at home !