The lemon battery experiment is one of the common experiments mentioned in science textbooks. A lemon battery consists of two metals suspended in an acidic solution. Copper and Zinc work well as metals for the battery and the citric acid present in the lemon act as an acidic solution. Batteries like these cannot run a motor or power up light bulbs, but it can produce a dim glow in LEDs. In this article, you will learn how to make a lemon battery and understand how a lemon battery works.
What is a Lemon Battery?
A lemon battery is a simple battery made using a zinc metal like a galvanized nail and a copper piece like a penny for educational purposes. These are inserted into a lemon and are connected by wires. The zinc and copper are called electrodes and lemon juice is an electrolyte.
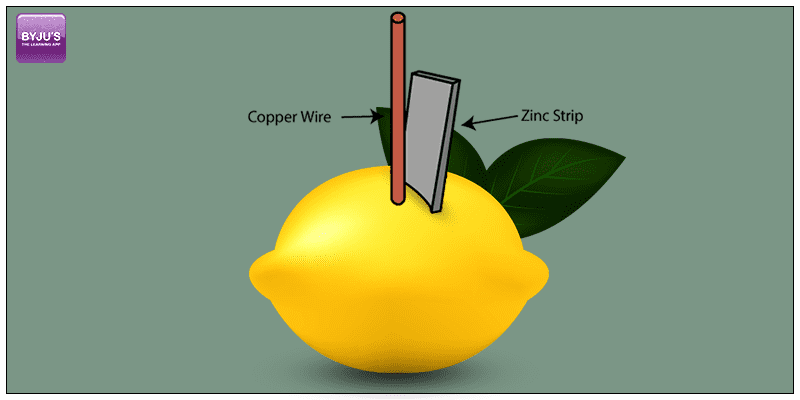
Lemon Battery
How To Make A Lemon Battery?
The lemon battery experiment listed here is similar to the experiment of the first electrical battery, invented by Alessandro Volta in the year 1800, where he used a brine solution. Listed below is a methodical explanation of the experiment.
Objective:
The functioning of a Lemon Battery
Things you will need:
- Citrus fruits (Lemon will be best)
- Copper wire of 18 gauge or lesser
- Wire-cutter/stripper
- A Zinc piece, small nail, Steel clips as an electrical conductor
Procedure:
- Strip around two and a half inches of plastic off the wire and cut that section away from the roll of copper wire you have.
- Take the paper clip and straighten it out. Using the clippers cut it to the same length as the wire of copper.
- Rub off any of the rough spots that are on your conductors. The end of the wire must be smooth as you will touch that to your tongue.
- The lemon needs to be pressed so that its cell walls breakdown and the juice is released. For the chemical reaction to take place the sour juices of lemon should be properly released.
- Take the copper wire and stick it for about an inch inside the lemon.
- Take the two free ends and stick them to your tongue. What do you notice?
You may also want to check out these topics given below!
What happens in a Lemon Battery?
When both the ends of the conductors touch the tongue of the person, he/she feels a slight tingle which was a small amount of current generated by the lemon. Your tongue feels the electrons moving through it. Electrons are the particles that revolve around the center of an electron and make up the portion that holds the negative charge.
The battery that we created is termed as a voltaic battery. This sort of battery is created out of two varying metals that do the work of electrodes or points of transfer for electrons. The salt present in your saliva makes it a conductor and the citric acid present in the sour lemon juice does the same in making electricity easy to flow.
What is an Electric Cell?



Comments