Table of Contents
- What is Diethyl Ether?
- General Properties of Diethyl Ether – (C2H5)2O
- Diethyl Ether Structure – (C2H5)2O
- Synthesis of Diethyl Ether – (C2H5)2O
- Physical Properties of Diethyl Ether – (C2H5)2O
- Chemical Properties of Diethyl Ether – (C2H5)2O
- Uses of Diethyl Ether – (C2H5)2O
- Health Hazard
- Frequently Asked Questions
What is Diethyl Ether?
Ether is a common name for diethyl ether. Ether is a volatile, flammable, colourless liquid with a distinctive odour. It belongs to the large group of organic compounds called ethers. It is also known by the name ethyl ether.
The chemical formula for diethyl ether is C2H5OC2H5. It is the most common ether known historically. The discovery of ether is credited to the German physician and botanist Valerius Cordus in 1515-1554.
General Properties of Diethyl Ether – (C2H5)2O
| C2H5OC2H5 | Diethyl Ether |
| Density | 713 kg/m³ |
| Molecular Weight/ Molar Mass | 74.12 g/mol |
| Melting Point | -116.3 °C |
| Boiling Point | 34.6 °C |
| Compound Formula | (C2H5)2O |
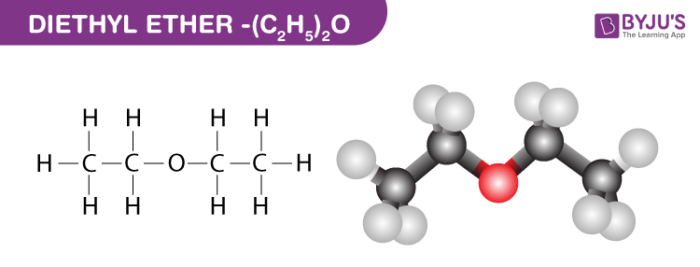
Diethyl Ether Structure – (C2H5)2O
Synthesis of Diethyl Ether – (C2H5)2O
Ether is synthesised by the dehydration of ethanol using sulphuric acid. The chemical reaction is as follows.
2CH3CH2OH + 2H2SO4 → (CH3CH2)2O + H2SO4 + H2O
Physical Properties of Diethyl Ether – (C2H5)2O
| Odour | Sweet, Pungent odour |
| Appearance | Colourless liquid |
| Vapour Pressure | 439.8 mm Hg at 20 deg C |
| Solubility | Ethers are polar in nature, so they are more soluble in water than alkanes. |
Chemical Properties of Diethyl Ether – (C2H5)2O
-
- Combustion – Ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water.C2H5OC2H5 + 6O2 → 4CO2 + 5H2O
- Halogenation – Ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight.
C2H5OC2H5 + Cl2 → C2H4(Cl)OC2H4(Cl)
Uses of Diethyl Ether – (C2H5)2O
- Used as a common laboratory solvent.
- Used as an excellent solvent for alkaloids, dyes, fats, oils, resins and waxes.
- Used in the recovery of acetic acid from aqueous solutions in cellulose acetate and plastic industry.
Health Hazard
Diethyl ether inhalation may cause headache, nausea, vomiting and loss of consciousness. If it comes in contact with skin or eyes it causes irritation. High exposure of diethyl ether can cause damage to the kidney.
Frequently Asked Questions
What is soluble in diethyl ether?
Ethers such as diethyl ether dissolve a wide variety of organic compounds of polar and non-polar origin. In diethyl ether, non-polar compounds are usually more soluble than alcohols because ethers do not have a hydrogen bonding network that needs to be broken up to dissolve the solute.
Is diethyl ether the same as ether?
Ethyl ether, also known as diethyl ether, well-known anaesthetic, generally referred to as simply ether, an organic compound belonging to a large group of compounds called ether; its molecular structure consists of two ethyl groups connected by an oxygen atom, as in C2H5OC2H5.
What happens if you inhale diethyl ether?
Breathing Diethyl Ether can cause nose and throat irritation. Breathing Diethyl Ether can cause drowsiness, arousal, dizziness, vomiting, irregular breathing, and saliva. High exposure may result in unconsciousness or even death.
Is ether still used as an Anaesthetic?
Thanks to its low cost and high therapeutic index with reduced heart and respiratory distress, ether is still used as an anaesthetic in several developing countries. The volatile flammability has done away with its use in most developing countries.
Is diethyl ether flammable?
Diethyl ether is highly flame retardant and can form explosive mixtures of air / vapour. Ether peroxides have a boiling point higher than ether, and are explosives of contact when dry.

Comments