What is Dioxygen?
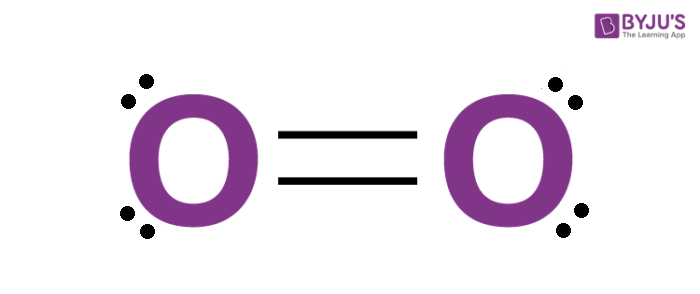
Dioxygen plays a vital part in the existence of life. It is a highly reactive non-metal, and it is a member of the chalcogen group on the modern periodic table. Oxygen is a diatomic molecule and hence two atoms of the elements combine to form dioxygen. It is the third most abundant element in the universe.
Around 21% of the earth’s atmosphere consists of this gas in its free state. The chemical formula of oxygen is O2. It is the most abundant element found in the earth’s crust.
Recommended Videos

Laboratory Preparation of Oxygen
In the laboratory, oxygen can be prepared in multiple ways:
Hydrogen peroxide in the presence of finely divided metals and manganese dioxide decomposes to give water and dioxygen.
Oxides of few metals decompose in the presence of heat to give O2
Salts of chlorates, permanganates and nitrates decompose on heating to give O2
Industrial preparation of O2
O2 is obtained on a large scale from the air. Carbon dioxide and water are removed from the air, and the remaining gases are eliminated by fractional distillation and liquefaction, which releases dinitrogen and O2.
Properties of Dioxygen
It is a colourless, odourless diatomic gas and paramagnetic in nature.
Oxygen is highly reactive non-metal.
This diatomic gas is a strong oxidizing gas.
It is the second most electronegative element after fluorine.
Dioxygen reacts with metals, and non-metals to give oxides of the respective element.
Uses of Dioxygen
Oxygen is used in many applications. Oxygen is used:
- As an oxygen supplement in medicine.
- For respiration purposes.
- In the industries for the smelting of iron ore to form steel.
- In metal cutting and welding.
- As an oxidizer in water treatment and rocket fuel.
Frequently Asked Questions – FAQs
Define molecule.
Is oxygen a diatomic or monoatomic molecule?
Write two uses of oxygen.
1. It is used in metal cutting and welding.
2. As an oxidizer in water treatment and rocket fuel.
Oxygen is a homonuclear or heteronuclear molecule?
For a detailed discussion on the preparation, properties and uses of dioxygen, please visit BYJU’S.


Its simple use of language makes it easy to digest this content on Di-oxygen.