What is Potassium Dichromate?
Potassium dichromate or anhydrochromate is prepared by adding to the neutral yellow chromate of potassium in solution, a moderate quantity of one of the stronger acids.
Potassium permanganate is commercially prepared by mixing a solution of potassium hydroxide and powdered manganese oxide with oxidizing agents like potassium chlorate.
Table of Contents
- Recommended Videos
- Preparation of Potassium Dichromate
- Structure of Potassium Dichromate Molecules
- Preparation of Potassium Permanganate
- Properties of K2Cr2O7 & KMnO4
- Applications of Potassium Dichromate
- Frequently Asked Questions – FAQs
Recommended Videos

Preparation of Potassium Dichromate – K2Cr2O7
- Potassium dichromate is an important chemical used in industries as an oxidizing agent and for the preparation of many other compounds.
- Dichromates are usually prepared from chromates and this is obtained by the combination of chromite ore with sodium/potassium carbonate in the presence of air.

Potassium Dichromate
The reaction can be given as:
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
The solution of sodium chromate (Na2CrO4) is further purified with sulphuric acid to form a solution from which the crystals of orange coloured sodium dichromate (Na2Cr2O7.2H2O) can be extracted.
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Now potassium dichromate can be obtained by reacting a solution of sodium dichromate with potassium chloride.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
Thus, we finally obtain the orange crystals of potassium dichromate.
Structure of Potassium Dichromate Molecules
Potassium dichromate is an ionic compound consisting of two potassium cations and one dichromate anion. The coordination geometries around the chromium atoms are tetrahedral. The structure of a potassium dichromate molecule is illustrated below.
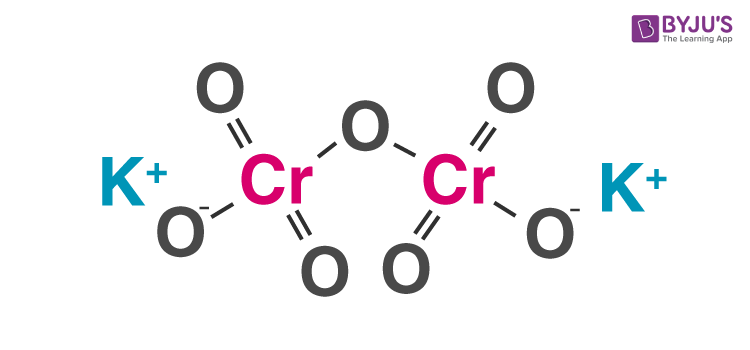
It can be noted that in the potassium dichromate molecule, potassium exhibits an oxidation state of +1, oxygen exhibits an oxidation state of -2 and chromium exhibits an oxidation state of +6. It can be noted that crystals of potassium dichromate have a triclinic structure.
Preparation of Potassium Permanganate – KMnO4
We can get this (KMnO4) by reacting MnO2 with an alkali metal hydroxide and KNO3 (oxidizing agent).
This will result in the production of dark green K2MnO4 which is disproportionate in an acidic or neutral medium to give permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42- + 4H+ → 2MnO4– + MnO2 + 2H2O
Therefore, the preparation of potassium permanganate involves a reaction of MnO2 with KOH to give MnO42- followed by electrolytic oxidation of manganate to give permanganate ion, MnO4–.
After this manganese ion salt gets oxidized by peroxodisulphate to permanganate ion as per the reaction given below.
2Mn2+ + 5S2O82- + 8H2O → 2MnO4– +10SO42- + 16H+
Thus the dark purple coloured crystals of potassium permanganate are obtained.
Properties of K2Cr2O7 & KMnO4
1. Properties of Potassium Dichromate, K2Cr2O7
- On heating, potassium dichromate decomposes to form potassium chromate, chromic oxide and oxygen.
4K2Cr2O7 → 4K2CrO4 + 2CrO3 + 3O2
- It is a powerful oxidizing agent. It oxidizes iodide to iodine.
Cr2O72- + 14H+ + 6I– → 2Cr3+ + 7H2O + 3I2
- The compound has bright red crystals and is used for dyeing, staining, tanning etc.
- For a medical purposes, it can be an astringent, antiseptic and caustic. It emits toxic chromium fumes when heated
- It is highly corrosive and is a strong oxidizing agent for which, it is used in wood preservatives, pigments manufacture and photochemical processes.
2. Properties of Potassium permanganate, KMnO4
- When potassium permanganate is heated it gives potassium manganate, manganese dioxide and oxygen.
2KMnO4 → K2MnO4 + MnO2 + O2
- It is a powerful oxidizing agent in acidic or alkaline solutions.
- It is in the form of purple crystals and gets soluble in hot water
- It is noncombustible but works as a catalyst in the burning of combustible materials.
- It will start a fire when mixed with glycerine.
- It can become toxic in high concentration
Applications of Potassium Dichromate
The primary application of K2Cr2O7 is in the preparation of potassium chrome alum, a compound which is used extensively in the tanning of leather. Chromic acid can also be prepared from this compound. Potassium dichromate is known to be used in the production of cement since it improves the texture and the density of the cement mixture.
Another important application of potassium dichromate is in the photography industry, where it is used in combination with a powerful mineral acid as an oxidizing agent for photographic screen printing. Since it is non-hygroscopic in nature, this compound is also employed for several wet tests in the field of analytical chemistry.
Frequently Asked Questions – FAQs
What is the use of potassium dichromate?
It is used in many applications as an oxidizing agent and is also used in the preparation of different products such as waxes, paints, glues, etc. Potassium dichromate is carcinogenic and highly toxic as a compound of hexavalent chromium.
What does the potassium dichromate test for?
For organic chemistry, potassium dichromate is an oxidizing agent that is milder than potassium permanganate. It is used for the oxidation of alcohol. This converts primary alcohols into aldehydes and carboxylic acids under more pressing conditions.
Is potassium dichromate light-sensitive?
Clear, light-sensitive orange crystals. Potassium dichromate is used in cotton dyeing as chromium mordant. In black and white image processing, potassium dichromate is used as an intensifier.
What is the charge of potassium dichromate?
K2Cr2O7 is the molecular formula. A reddish-brown colour as a solid and a molecular weight of 294.18 grams per mole is the physical properties of potassium dichromate. Potassium dichromate is also referred to as a compound of hexavalent chromium, and chromium oxidation is 6+.
Why KMnO4 is a self indicator?
So the solution loses its pink colour once all the permanganate ions are used up in the reaction. It means the end of the reaction and therefore a self-indicator is called potassium permanganate as it acts as an indicator apart from being one of the reactants.
Download BYJU’S – The Learning App and experience learning in an innovative way.

Comments