What is Zinc Oxide?
Zinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. It is naturally found as a mineral zincite. It is mostly produced synthetically. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. It has wide applications in bandages, ointments, pastes, and dental cement.
Table of Contents
- Physical Properties of Zinc Oxide
- Chemical properties of Zinc Oxide
- Properties of Zinc Oxide (ZnO)
- Structure Of Zinc Oxide (ZnO)
- Uses Of Zinc Oxide (ZnO)
- Effects on Health
Physical Properties of Zinc Oxide
Zinc white crystallises mainly in two forms viz cubic zinc blende and hexagonal wurtzite. The most common and stable structure under ambient conditions is wurtzite. Zincblende can be stabilised by growing zinc oxide on substrates which have a cubic lattice structure. The oxide and zinc centres are tetrahedral.
Chemical properties of Zinc Oxide
Calamine is a white solid which is insoluble in water and has no smell. Crude zinc oxide appears yellow-grey in colour and exists in a granular solid form with no odour. In its natural form, it is obtained as a mineral zincite which consists of manganese (Mn) and some other impurities which make it appears yellow-red colour. In its crystalline form, it is thermochromic which on heating in the presence of air changes its colour from white to yellow and on cooling it turns white in colour. It is an amphoteric oxide which is insoluble in water. It dissolves in acids and alkalis.
Properties of Zinc Oxide (ZnO)
| Zinc Oxide | ZnO |
| Molecular Weight of Zinc Oxide | 81.406 g/mol |
| Density of Zinc Oxide | 5.6 g/cm3 |
| Boiling Point of Zinc Oxide | 1,974 °C |
| Melting Point of Zinc Oxide | 1,974 °C |
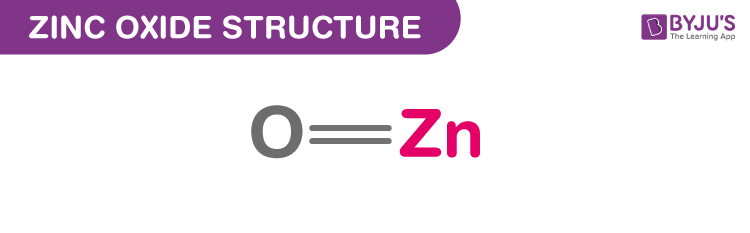
Structure Of Zinc Oxide (ZnO)
Uses Of Zinc Oxide (ZnO)
- Zinc oxide is used as a bulking agent.
- It is used as a colourant.
- ZnO is used in over-the-counter drug products.
- It is used as a skin protectant.
- It is used as a sunscreen as it reduces and prevents sunburn.
- It is used in preventing premature skin ageing.
- Used in nail products.
- It is used as a diaper rash ointment.
Effects on Health
People allergic to ZnO should not use products made of zinc oxide. It may lead to skin irritation, redness of the skin or worsening rashes.
Learn more about the chemical behaviour and importance of Zinc oxide (ZnO) with the expert faculties at BYJU’S.

Comments