What is Benzene hexachloride?
Benzene hexachloride is an isomer of hexachlorocyclohexane with a chemical formula C6H6Cl6. It is also known as Lindane or hexachlorane. Benzene hexachloride is a colourless solid with a slight musty odour. It is an organochlorine chemical and is widely used as an agricultural insecticide as well as a pharmaceutical treatment for scabies and lice. Some side effects of lindane are burning, stinging, or redness of the skin. In the year 1825, Faraday was the first person to originally synthesize this chemical. In the year 1942, a Dutch chemist Teunis van der Linden isolated Benzene hexachloride. He was the first one to describe γ-hexachlorocyclohexane in the year 1912. Its pesticidal action was discovered in 1942.
Table of Contents
- Preparation of Benzene hexachloride
- Properties of Benzene hexachloride – C6H6Cl6
- Benzene hexachloride structure – C6H6Cl6
- Uses of Benzene hexachloride (C6H6Cl6)
- Benzene hexachloride health risks
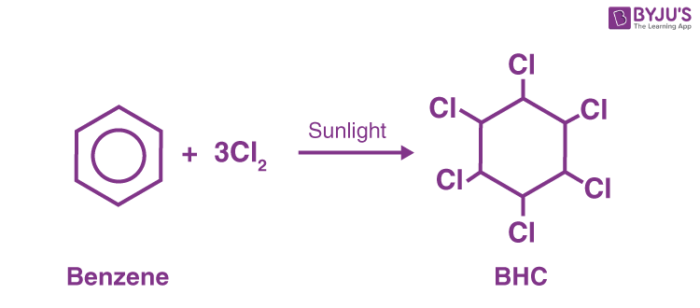
Preparation of Benzene hexachloride
- Chlorine combines with benzene, in the presence of sunlight and in the absence of oxygen as well as substitution catalysts, to form hexachlorocyclohexane.
- Lindane can be prepared from chlorine and benzene by photochlorination. The product obtained i.e benzene hexachloride comprises isomers from which only the gamma-isomer is wanted. Gamma-isomer is got by treating the reaction mixture with acetic acid or methanol in which only the alpha and beta isomers dissolve easily.
Properties of Benzene hexachloride – C6H6Cl6
| C6H6Cl6 | Benzene hexachloride |
| Molecular Weight/ Molar Mass | 290.814 g/mol |
| Density | 1.89 at 66°F |
| Boiling Point | 323°C |
| Melting Point | 113°C |
Benzene hexachloride structure – C6H6Cl6
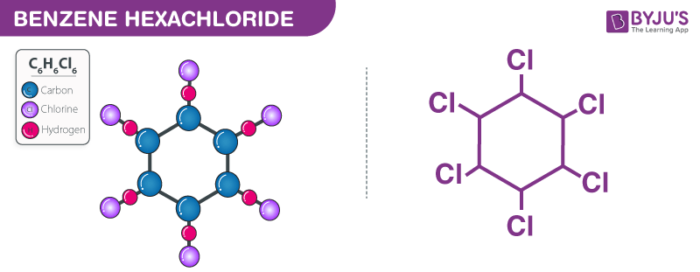
The above image describes the Benzene hexachloride structure. C6H6Cl6 is the chemical formula of Benzene hexachloride. The Benzene hexachloride molecule is a gamma-isomer which is expected to be a human carcinogen. Benzene hexachloride has six carbon atoms, six hydrogen atoms, and six chlorine atoms.
Uses of Benzene hexachloride (C6H6Cl6)
- Benzene hexachloride is used as an insecticide on crops, in forestry, for seed treatment.
- It is used in the treatment of head and body lice.
- It is used in pharmaceuticals.
- It is used to treat scabies.
- It is used in shampoo.
Benzene hexachloride health risks
It is highly toxic but non-combustible. Lindane can cause irritation on contact. When swallowed, inhaled, or absorbed through the skin it may be fatal. Better to avoid skin contact. When inhaled the effects will be delayed. Fire produces irritation, toxic, and corrosive gases. This compound is a stimulant of the nervous system, which causes violent convulsions that are rapid in onset and lead to death or recovery within 24 hours of time.
To learn more about the structural details and chemical reactions of Benzene hexachloride (C6H6Cl6) from the expert faculties at BYJU’S register now!
Other important links:
| Isomerism | Combustion Types |


Comments