What is the Carbon Atom?
The name of Carbon comes from the Latin word carbo, which means “coal”. It is a chemical element with the symbol C and the atomic number 6. It is nonmetallic and tetravalent, which means it can form covalent chemical bonds with four electrons. It is in Periodic Group 14 of the periodic table. Carbon accounts for only about 0.025 %of the Earth’s crust. Natural isotopes include 12C and 13C, which are stable, and 14C, which is a radionuclide with a half-life of about 5,730 years.
Carbon is the 15th most abundant element in the Earth’s crust and, by mass, the fourth most abundant element in the universe. Carbon’s abundance, the unique diversity of organic compounds, and unusual ability to form polymers at common Earth temperatures allow it to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5 %).

Table of Contents
- What are Protons?
- Number of Protons in the Carbon Atom
- Atomic Mass of Carbon Atom
- Uses of Carbon Atom
- Frequently Asked Questions – FAQs
What are Protons?
Protons are positively charged particles found in the nucleus of a hydrogen atom. A proton weighs 1.6726219 × 10-27 kilograms.
Protons, electrons, and neutrons make up an atom. The nucleus, which is located in the centre of an atom, contains the entire mass of the atom. The nucleus is made up of protons and neutrons, which are referred to collectively as nucleons.
Number of Protons in the Carbon Atom
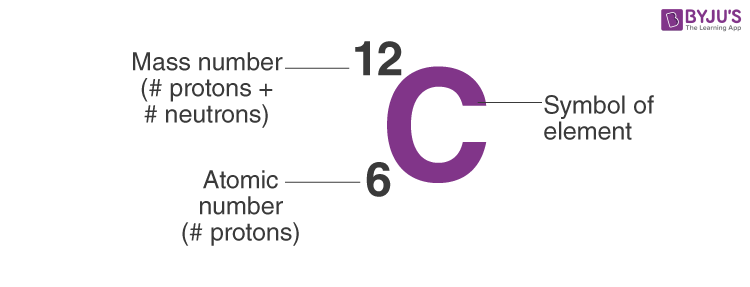
- A Carbon-12 neutral atom has six protons, six neutrons, and six electrons.
- Carbon – 14 is a neutral element with six protons, eight neutrons, and six electrons.
Some isotopes are stable, but others can emit or kick out subatomic particles in order to achieve a more stable, lower-energy configuration. These isotopes are referred to as radioisotopes, and the process by which they emit particles and energy is referred to as decay. When the number of protons in the nucleus changes due to radioactive decay, the identity of the atom changes. This phenomenon can be observed in the Carbon – 14 isotope.
- Carbon-14 decays to nitrogen-14
Half of the carbon-14 that was initially present will have been converted to nitrogen-14 after a half-life of approximately 5,730 years.
Atomic Mass of Carbon Atom
Carbon-12, a carbon atom with six neutrons, has an atomic mass of 12 amu (6 protons + 6 neutrons).
The carbon isotope C-14 with six protons and eight neutrons in its nucleus. This results in an atomic mass of 14 amu (6 protons + 8 neutrons).
Uses of Carbon Atom
- Carbon is used as a fuel (in the form of coal, which is mostly carbon).
- Carbon can combine with iron to form alloys, the most common of which is carbon steel.
- Archaeological dating relies on the radioactive isotope C-14.
- It is used to make sugar, glucose, proteins, and other substances. Carbohydrates, which are elements of carbon, are an important source of energy in the food we eat.
- Carbon, in the form of diamond, is used in jewellery and is the hardest substance known. However, diamonds are also used in industries for cutting, grinding, drilling, and polishing procedures.
- Amorphous carbon is used in the production of inks and paints. It’s also found in batteries.
- The lead in pencils is made of graphite. It is also used in the manufacture of steel.
- Carbon compounds play an important role in many aspects of the chemical industry because carbon can form a wide range of compounds with hydrogen, oxygen, nitrogen, and other elements.
Frequently Asked Questions on Carbon Protons
Does carbon have 4 protons?
The atomic number of carbon is 6. That means a carbon atom has six protons, six neutrons, and six electrons.
How do you find protons?
The number of protons in an atom equals the element’s atomic number.
What is the number of protons in carbon dioxide?
Each oxygen atom contains eight protons, eight electrons, and eight neutrons. As a result, the CO2 molecule contains a total of 22 protons, 22 electrons, and 22 neutrons.
How many isotopes of carbon are there?
There are three isotopes of Carbon. They are Carbon – 12, Carbon – 13 and Carbon – 14.
Does carbon have 12 protons?
No, carbon has 6 protons. The mass of C – 12 is 12 amu.

Comments