A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction.
Table of Content
What is a Fuel Cell?
Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. Therefore, these cells can constantly generate electricity until the supply of fuel and oxygen is cut off.
Despite being invented in the year 1838, fuel cells began commercial use only a century later when they were used by NASA to power space capsules and satellites. Today, these devices are used as the primary or secondary source of power for many facilities including industries, commercial buildings, and residential buildings.
A fuel cell is similar to electrochemical cells, which consists of a cathode, an anode, and an electrolyte. In these cells, the electrolyte enables the movement of the protons.
Recommended Videos
Working of Fuel Cell
The reaction between hydrogen and oxygen can be used to generate electricity via a fuel cell. Such a cell was used in the Apollo space programme and it served two different purposes – It was used as a fuel source as well as a source of drinking water (the water vapour produced from the cell, when condensed, was fit for human consumption).
The working of this fuel cell involved the passing of hydrogen and oxygen into a concentrated solution of sodium hydroxide via carbon electrodes. The cell reaction can be written as follows:
Cathode Reaction: O2 + 2H2O + 4e– → 4OH–
Anode Reaction: 2H2 + 4OH– → 4H2O + 4e–
Net Cell Reaction: 2H2 + O2 → 2H2O
However, the reaction rate of this electrochemical reaction is quite low. This issue is overcome with the help of a catalyst such as platinum or palladium. In order to increase the effective surface area, the catalyst is finely divided before being incorporated into the electrodes.
A block diagram of this fuel cell is provided below.
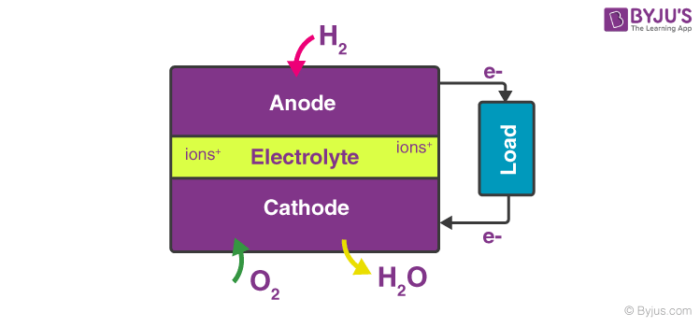
The efficiency of the fuel cell described above in the generation of electricity generally approximates to 70% whereas thermal power plants have an efficiency of 40%. This substantial difference in efficiency is because the generation of electric current in a thermal power plant involves the conversion of water into steam, and the usage of this steam to rotate a turbine. Fuel cells, however, offer a platform for the direct conversion of chemical energy into electrical energy.
Types of Fuel Cells
Despite working similarly, there exist many varieties of fuel cells. Some of these types of fuel cells are discussed in this subsection.
The Polymer Electrolyte Membrane (PEM) Fuel Cell
- These cells are also known as proton exchange membrane fuel cells (or PEMFCs).
- The temperature range that these cells operate in is between 50oC to 100oC
- The electrolyte used in PEMFCs is a polymer which has the ability to conduct protons.
- A typical PEM fuel cell consists of bipolar plates, a catalyst, electrodes, and the polymer membrane.
- Despite having eco-friendly applications in transportation, PEMFCs can also be used for the stationary and portable generation of power.
Phosphoric Acid Fuel Cell
- These fuel cells involve the use of phosphoric acid as an electrolyte in order to channel the H+
- The working temperatures of these cells lie in the range of 150oC – 200oC
- Electrons are forced to travel to the cathode via an external circuit because of the non-conductive nature of phosphoric acid.
- Due to the acidic nature of the electrolyte, the components of these cells tend to corrode or oxidize over time.
Solid Acid Fuel Cell
- A solid acid material is used as the electrolyte in these fuel cells.
- The molecular structures of these solid acids are ordered at low temperatures.
- At higher temperatures, a phase transition can occur which leads to a huge increase in conductivity.
- Examples of solid acids include CsHSO4 and CsH2PO4 (cesium hydrogen sulfate and cesium dihydrogen phosphate respectively)
Alkaline Fuel Cell
- This was the fuel cell which was used as the primary source of electricity in the Apollo space program.
- In these cells, an aqueous alkaline solution is used to saturate a porous matrix, which is in turn used to separate the electrodes.
- The operating temperatures of these cells are quite low (approximately 90oC).
- These cells are highly efficient. They also produce heat and water along with electricity.
Solid Oxide Fuel Cell
- These cells involve the use of a solid oxide or a ceramic electrolyte (such as yttria-stabilized zirconia).
- These fuel cells are highly efficient and have a relatively low cost (theoretical efficiency can even approach 85%).
- The operating temperatures of these cells are very high (lower limit of 600oC, standard operating temperatures lie between 800 and 1000oC).
- Solid oxide fuel cells are limited to stationary applications due to their high operating temperatures.
Molten Carbonate Fuel Cell
- The electrolyte used in these cells is lithium potassium carbonate salt. This salt becomes liquid at high temperatures, enabling the movement of carbonate ions.
- Similar to SOFCs, these fuel cells also have a relatively high operating temperature of 650oC
- The anode and the cathode of this cell are vulnerable to corrosion due to the high operating temperature and the presence of the carbonate electrolyte.
- These cells can be powered by carbon-based fuels such as natural gas and biogas.
Applications of fuel cell
Fuel cell technology has a wide range of applications. Currently, heavy research is being conducted in order to manufacture a cost-efficient automobile which is powered by a fuel cell. A few applications of this technology are listed below.
- Fuel cell electric vehicles, or FCEVs, use clean fuels and are therefore more eco-friendly than internal combustion engine-based vehicles.
- They have been used to power many space expeditions including the Appolo space program.
- Generally, the byproducts produced from these cells are heat and water.
- The portability of some fuel cells is extremely useful in some military applications.
- These electrochemical cells can also be used to power several electronic devices.
- Fuel cells are also used as primary or backup sources of electricity in many remote areas.
Frequently Asked Questions – FAQs
What is a fuel cell?
A fuel cell is an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. It offers high efficiency and zero emissions.
How does a fuel cell differ from conventional methods of energy generation?
A fuel cell is different from the conventional methods of energy generation because, in a fuel cell, chemical energy is directly converted into electrical energy without intermediate conversion into mechanical power.
Why is fuel cell better than the conventional methods of energy generation?
A fuel cell is preferred over conventional methods of energy generation because, in a fuel cell, zero combustion takes place. Thus, carbon dioxide is not produced.
What are the benefits of a fuel cell?
Fuel cells provide clean energy and emit no pollution. Moreover, it also offers high efficiency and zero emissions. No carbon dioxide is produced while generating chemical energy from a fuel cell.
Which electrolyte is used in molten carbonate fuel cells?
Lithium potassium carbonate salt is used as an electrolyte in molten carbonate fuel cells.
Thus, the different types of fuel cells and the working of an alkaline fuel cell are briefly discussed in this article along with some applications of these electrochemical cells. To learn more about this technology and other related topics, register with BYJU’S and download the mobile application on your smartphone.

Comments