What is Hydrogen Peroxide?
Hydrogen peroxide is a hydrogen-oxygen chemical compound. Anhydrous hydrogen peroxide is a colourless, syrupy liquid that decomposes into oxygen and water very easily.
H2O2 (hydrogen peroxide) is a colourless liquid that is similar to water in several ways. It has physical properties that are very similar to water, with the exception that it is 40% denser. However, the chemical behaviour of hydrogen peroxide and water differs significantly.
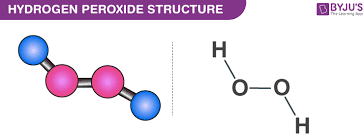
The form of hydrogen peroxide H2O2 is H—O—O—H, with each dash reflecting a single covalent bond. That is, each bond contains a pair of mutual electrons, formally one from each of the atoms at the bond’s ends. The H—O bonds are polar, while the O—O bonds are not.
Related Topics
Hydrogen Peroxide Structure
Hydrogen Peroxide as Disinfectant
Germs, like most viruses and bacteria, are destroyed by hydrogen peroxide. A disinfectant with a concentration of 3% hydrogen peroxide is commonly available in supermarkets. Be careful when handling hydrogen peroxide because it can destroy certain surfaces and is a more harmful chemical than some disinfectants.
Currently available on the market When used on inanimate surfaces, 3 percent hydrogen peroxide is a stable and efficient disinfectant. Another popular household commodity, hydrogen peroxide, can be used to destroy bacteria and viruses. It should be combined with water and washed down in the same way as bleach H2O2 can stain clothes and porous counters, so use it with caution.
Hydrogen Peroxide as Bleaching Agent
H2O2 can also be used as a bleaching agent. Because of its oxidizing properties, H2O2 can react directly with double bonds in large organic molecules, forming organic peroxides. H2O2 is used to bleach wood pulp for white paper and the melanin in hair in this way.
As a bleaching agent for secondary fibers, hydrogen peroxide is appealing. When high levels of brightness are needed, hydrogen peroxide is the most commonly used chemical for high-yield pulp bleaching.
The key drawback is that hydrogen peroxide has a limited disinfecting and oxidizing capacity at active concentrations, which are required for swimming pool disinfection. Another issue is hydrogen peroxide’s rapid decomposition in water and the presence of oxygen radicals.
Hydrogen Peroxide Uses for Plants
Once a week, or after it rains, water mature plants with the hydrogen peroxide solution. Combine equal parts distilled water and 3% hydrogen peroxide in a mixing bowl. Soak the contaminated plants and the area around them thoroughly with a spray bottle.
Hydrogen peroxide can be used to grow a wide range of plants in hydroponic gardens, raised beds, and greenhouses. It functions as an oxygen substitute for plants by releasing oxygen. It appears to be extremely beneficial to plant health and development. Hydrogen peroxide may also aid in the treatment of soil fungus.
Hydrogen peroxide assists in the increase of oxygen levels in water. It will also assist in the removal of any bacteria that causes mold or fungus. Fungus gnat eggs and larvae will be killed by this process. The solution will also assist in the addition of oxygen to the soil of your indoor plants, as well as the removal of any other bacterial or fungal pests.
Hydrogen Peroxide used in Dentistry
In today’s dentistry, hydrogen peroxide is used safely and efficiently. Though tooth whitening is the most popular use, hydrogen peroxide has been shown to have substantial health benefits when used to treat gingivitis and periodontitis.
Gargling hydrogen peroxide can help to relieve a sore throat, disinfect the mouth, and whiten the teeth. Hydrogen peroxide can aid in the treatment of gum disease due to its antibacterial properties. A biofilm is a slimy film of bacteria that develops on the teeth as plaque forms. Hydrogen peroxide produces oxygen, which aids in the destruction of bacteria.
The soft tissues within the mouth are not protected when using high concentrations of hydrogen peroxide teeth whitening gel. In a painful reaction to this hard gel, the gums will turn white and blister. Fortunately, these wounds are only temporary and heal easily.
Frequently Asked Questions – FAQs
Can hydrogen peroxide whiten teeth?
Hydrogen peroxide is a popular household item that you probably already have on hand. It can be an easy way to whiten your teeth if used correctly. However, if used improperly — at too high concentrations or too often — it can cause severe and sometimes costly tooth damage.
Is hydrogen peroxide safe to use?
Diluted hydrogen peroxide products, which usually contain around 3% hydrogen peroxide, are safe to use in the home on a regular basis. If not treated correctly, hydrogen peroxide in more concentrated forms, such as solutions containing 30% hydrogen peroxide, can be dangerous.
Is hydrogen peroxide rubbing alcohol?
Hydrogen peroxide, unlike isopropanol, is not an alcohol. You can recognize H2O2 as a chemical formula that is similar to that of water (H2O). Hydrogen peroxide differs from water in that it contains two oxygen molecules rather than one. It’s a strong oxidizer because of the extra oxygen molecule.
What is a hydrogen peroxide example?
Hair dyes and bleaches, toothpaste and mouthwashes, bathroom cleaners, and laundry stain removers all contain hydrogen peroxide, a colourless solvent that is used in a number of cleaning and personal care items.
Is hydrogen peroxide a good antibacterial?
Hydrogen peroxide is a common antimicrobial agent. It’s used as a preservative, disinfectant, and sterilizer in both liquid and gas type. Its benefits include potent and broad-spectrum antimicrobial activity, versatility in application, and a low risk profile compared to other microbicides.
Is hydrogen peroxide better than bleach?
Since hydrogen peroxide isn’t as powerful as bleach, it’s less likely to harm fabrics, but it may discolour them, according to Sachleben. Using it straight, without diluting it. Water and oxygen are formed as hydrogen peroxide decomposes.
Comments