What are Carbon and its Compounds?
The molecules of a carbon compound must contain an atom of carbon. All living things, including plants and animals, are composed of organic components, which are carbon-based substances.
Carbon compounds are present everywhere i.e. in the food that we eat, the clothes that we wear and even in the lead of the pencil by which we write. The atomic number of carbon is 6 and the atomic mass is 12.01gmol-1. Carbon is a member of the 14th group. According to the data, it is the seventeenth most abundant element found on Earth. It is found in both free as well as in the combined state.
It is found in both free as well as in the combined state. It can be available as coal, graphite in the elemental state. Whereas it is present as metal carbonates, hydrocarbons, and carbon dioxide gas in the combined state. When it combines with other elements such as dihydrogen, dioxygen, chlorine, and sulphur provides amazing arrays of materials that can vary from tissues to medicines.
Table of Content |
Catenation Property of Carbon
In Organic Chemistry, everything is surrounded by carbon compounds. It is one of the most essential components of living organisms. There are two stable isotopes of carbon 12C and 13C. After these two one more isotope of carbon is present 14C. Carbon is used for radiocarbon dating and it is also a radioisotope with a half-life of 5770 years.
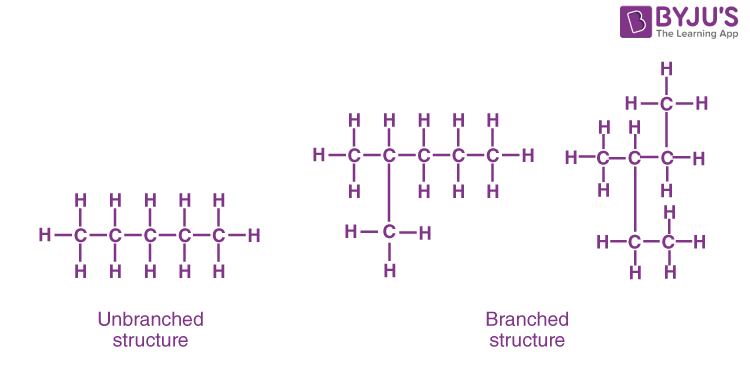
- One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation.
- Carbon has many special abilities out of all one unique ability is that carbon forms pπ-pπ bonds which are nothing but double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen.
- Just because of these two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms.
- Carbon atoms form tetravalent bonds , so that one carbon atom forms a bond with four carbon atoms and this structure can be repeated endlessly without disturbing the stability of the bonds.
For more information you may visit : Catenation
Recommended Videos of Carbon and its Compounds
Carbon and Its Compounds

Important Questions & Problems

Carbon and Its Compounds in One-Shot

Carbon and Its Compounds Important Questions

Understanding Carbon
Fullerenes
Do you know what the allotrope of carbon means?
Allotrope is nothing but the existence of an element in many forms which will have different physical properties but will have similar chemical properties and its forms are called allotropes of allotropic forms. Allotropes are defined as the two or more physical forms of one element. These allotropes are all based on carbon atoms but exhibit different physical properties, especially with regard to hardness.
The common, crystalline allotropes of carbon are Diamond, Graphite, Graphene, Fullerene ( C60), and Carbon nanotubes. Carbon shows allotropy because it exists in different forms of carbon such as diamond, and graphite. But it is now known that all the amorphous carbons contain microcrystals of graphite. Though these allotropes of carbon have different crystal structures and different physical properties, their chemical properties are the same and show similar chemical properties. Both diamond and graphite have the symbol C. Both give off carbon dioxide when strongly heated in the presence of oxygen.
The structure of carbon allotropes are
Diamond:
Diamond is a well known allotrope of carbon having a three-dimensional network structure. It exhibits the highest hardness and thermal conductivity.
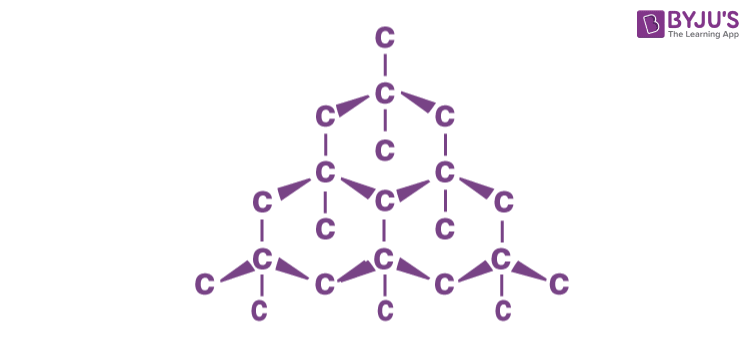
Graphite:
Graphite is another allotrope of carbon. It is a two dimensional structure and a good conductor of electricity.
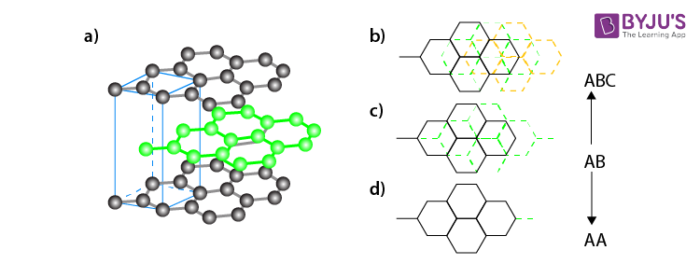
Graphene:
It is a single layer allotrope of carbon having a two dimensional honeycomb structure. Graphene has very high electron mobility and, like graphite, is a good electrical conductor, due to the occurrence of a free pi (p) electron for each carbon atom.
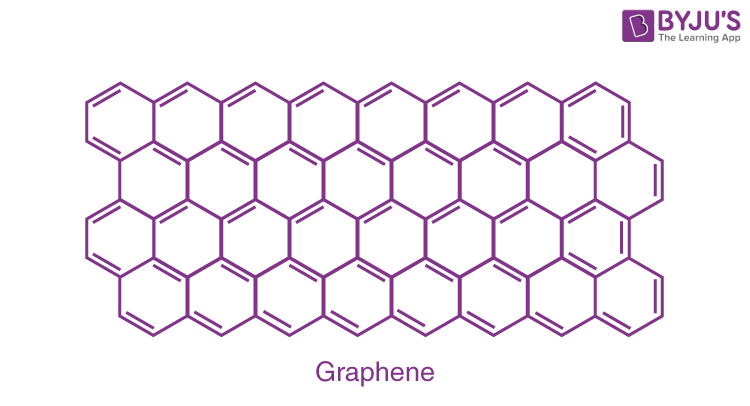
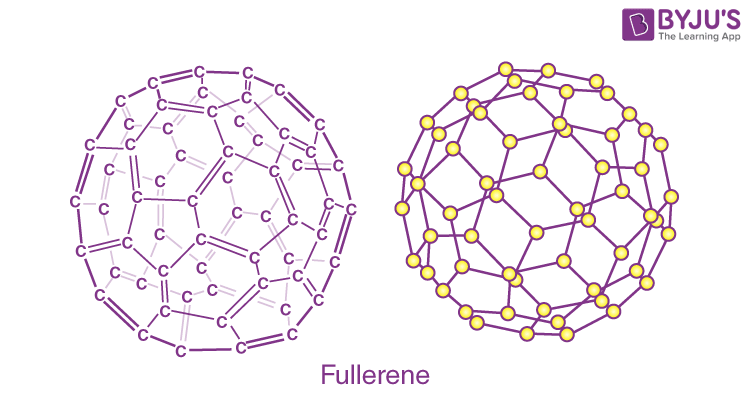
Fullerene (C6O):
A fullerene is an allotrope of carbon atoms connected by single and double bonds to form a closed or partially closed structure, with fused rings of five to seven atoms.
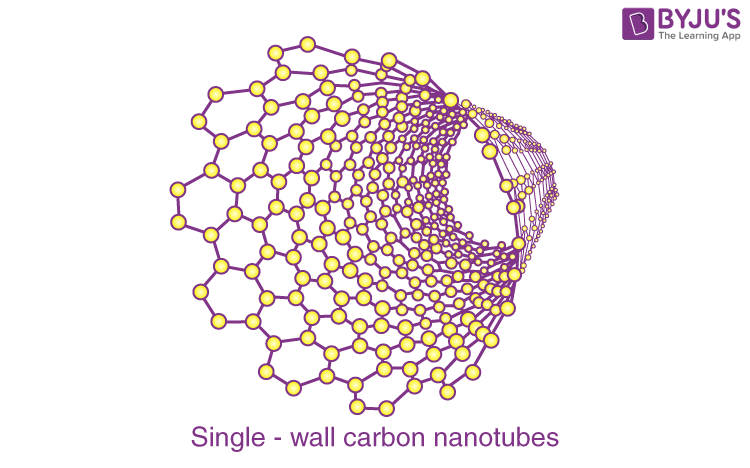
Single-wall carbon nanotubes:
Single-wall carbon nanotubes are one of the allotropes of carbon, intermediate between fullerene cages and flat graphene.
Some Carbon based compounds
Carbon -hydrogen compounds:
Hydrocarbons are the compound of carbon having a carbon hydrogen bond. Such as CH4, C2H6, C2H4, C6H6 etc.
Carbon-oxygen compounds:
There are many oxides of carbon compounds there such as carbon dioxide (CO2), carbon monoxide (CO), carbon trioxide (CO3) etc.
Carbon-sulfur compounds:
The carbon-sulphide compounds are carbon disulphide (CS2) and carbonyl sulphide (OCS). Carbon monosulphide (CS) etc.
Carbon-nitrogen compounds
The carbon-nitrogen compounds are cyanogen (CN)2 , hydrogen cyanide (HCN), cyanogen chloride (CNCl) etc.
Carbon- halides compounds
The common carbon halides are carbon tetrafluoride (CF4), carbon tetrachloride (CCl4), carbon tetrabromide (CBr4), carbon tetraiodide (CI4) etc
Uses of carbon and its compounds
- Carbon is the basic building block of life.
- Hydrocarbons are the compounds of carbon and hydrogen are the major source of fuel.
- Carbon based compounds like ethylene, polypropene, polystyrene etc. are widely used in polymerisation process.
- They are used in the synthesis of many dyes and drugs.
- Diamond is a allotrope of carbon used for cutting marble, granite and glass, it is also used for jewellery.
- Graphite is used to make electrodes for electrolytic cells, for making pencils, lubricates for machines.
- Glucose is made of carbon , hydrogen and oxygen used is the main source of fuel for our cells
Frequently Asked Questions – FAQs
Why is carbon so important?
Is everything made out of carbon?
What are the everyday uses of carbon?
What are the 4 types of carbon compounds?
Which is the purest form of carbon?
The best way to get a better understanding of Carbon and its Compounds is to refer to NCERT solutions. BYJU’S provides NCERT solutions for Carbon and its Compounds.

Comments