What Is Karl Fischer Titration?
Karl Fischer titration is a titration method that uses volumetric or coulometric titration to determine the quantity of water present in a given analyte. This method for quantitative chemical analysis was developed by the German chemist Karl Fischer in the year 1935, Today, specialized titrators (known as Karl Fischer titrators) are available to carry out such titrations.
Principle of Karl Fischer Titration
The principle of Karl Fischer titration is based on the oxidation reaction between iodine and sulphur dioxide. Water reacts with iodine and sulphur dioxide to form sulphur trioxide and hydrogen iodide. An endpoint is reached when all the water is consumed. The chemical equation for the reaction between sulphur dioxide, iodine, and water (which is employed during Karl Fischer titration) is provided below.
I2 + SO2 + H2O → 2HI + SO3
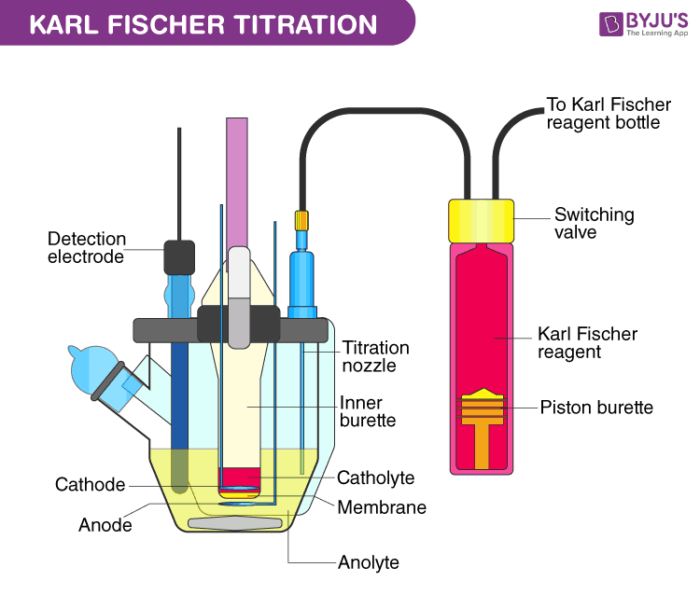
Karl Fischer Titration Equipment
Drying tube, sample injection cap, electrode analysis, Drain cook, a cathode chamber, detection electrode, rotor, anode chamber, KF reagent.
Ingredients of KF reagent:
Iodine, Buffer (Imidazole), sulphur dioxide, solvent (methanol).
Karl Fischer Titration Procedure
The Karl Fischer titration experiment can be performed in two different methods. They are:
- Volumetric determination – This technique is suitable to determine water content down to 1% of water. The sample is dissolved in KF methanol and the iodine is added to KF Reagent. The endpoint is detected potentiometrically.
- Coulometric determination – The endpoint is detected in this experiment electrochemically. Iodine required for KF reaction is obtained by anodic oxidation of iodide from solution.
Karl Fischer Titration Applications
- It is used in technical products such as plastics, oils, gases.
- It is used in pharmaceutical products.
- It is used in cosmetic products.
- It is used in the industry.
Advantages of Karl Fischer Titration
- It is fitted for determining water in gases, liquids and solids.
- The coulometric titrator helps in detecting free water, dissolved water, and emulsified water.
- It is a swift process which demands a minimal amount of sample preparation.
- Extremely accurate method.
Limitations of Karl Fischer Titration
- It is a destructive technique.
- The solvent consumption is high as the manual volumetric titration demands reloading during each determination.
- Coulometric titration is fitted only for samples that contain a small amount of water.
- Coulometric titration takes extremely long periods to determine.
To learn more about the Karl Fischer Titration from the experts at BYJU’S, register now!
Frequently Asked Questions
What is the use of methanol in Karl Fischer titration?
Methanol is used as a solvent in Karl Fischer titrations.
How can the KF reagent be prepared?
Make a solution by mixing 170 mL of pyridine and 670 mL of methanol. Add 125 g of iodine to the solution and cool it. Take a 250 mL graduated cylinder and add 100 mL of pyridine. Keep it on an ice bath. Pass in sulphur dioxide (dry) till its volume reaches 200 mL.
What is a major difference between volumetric and coulometric titration?
The main difference between them is that:
Volumetric method – the titrant is directly added to the sample with the help of a burette.
Coulometric method – the titrant is produced electrochemically inside the titration cell.
What is coulometric Karl Fischer?
The Karl Fischer titration is merely a means of measuring sample water content. Modern instruments, such as the Aquamax KF, apply the coulometric principle, whereby the water present in the sample is coulometrically titrated to a predefined endpoint at which free iodine exceeds a minute.
Why we use sodium tartrate in Karl Fischer?
The volumetric standard for Karl Fischer titration is sodium tartrate dihydrate. It is stable and non-hygroscopic, under normal conditions. Sodium tartrate dihydrate has a 15.66 percent stoichiometric water content and is primarily used in volumetry to measure the titer.
What electrode is KF titration?
The reaction has reached its termination point because the iodine is in abundance. For electrochemical indication of the end point, the most complex KF titration technology uses a double platinum electrode, but visual and photometric indications are still used.
How do you calculate Karl Fischer factor?
The water equivalence factor F is determined according to the formula 0.1566 x w / v in mgs of H2O per ml of reagent, where W is the sodium tartrate weight in mgs, and V is the reagent volume in ml.
How do you make Karl Fischer reagent?
A solvent alcohol (ROH), an established concentration of iodine (I2), a base (RN) and sulphur dioxide (SO2) are the reagents. In an aqueous environment, the Bunsen reaction between iodine and sulphur dioxide is the basis for the reactions of Karl Fischer Reagents.
Other important links:
| Types of titration | Potentiometric titration |


why methanol only using for KF for find the water content in a substances
Since methanol is more hydrophilic than other alcohols and also polar, it dissolves substances more readily. Since most medications are polar, methanol is an excellent solvent for them.