Table of Contents
What are Micelles?
Micelles are formed by a cumulative formation of amphipathic molecules in an aqueous solution. In simple terms, it is formed when an array of solutions is added to water. The molecules can either be phospholipid or fatty acids. In a micelle, ionic heads form an outer shell in contact with water, while nonpolar tails are sequestered inside.
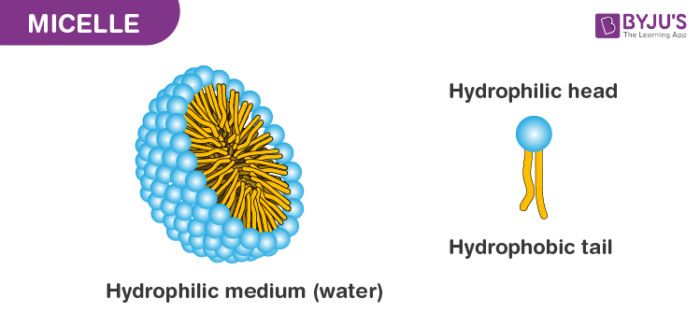
Therefore, the focal point of a micelle, being confined to long non-polar tails, takes after an oil or fuel drop. The length of the non-polar tail, the nature and size of the polar or ionic head, the sharpness of the plan, the temperature, and the closeness of included salts are essential factors in choosing the cumulative.
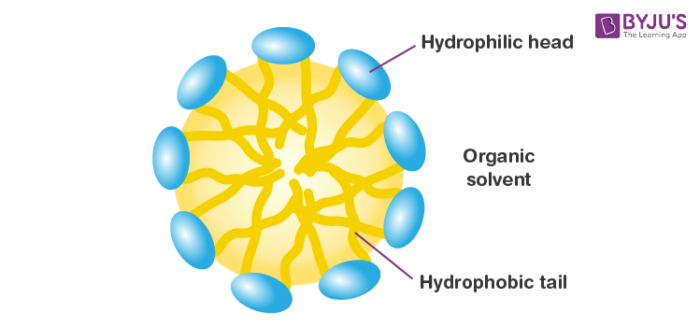
Reverse Micelle
Reverse micelles are water droplets that are obtained from the action of sulphates when dispersed in water. They are nanometer-sized. Surfactant atoms compose with the polar part to the internal side ready to solubilize water and the apolar part in contact with the natural dissolvable. Proteins can be solubilized in the water pool of switch micelles.
Studies on the structure-work connections of proteins in switch micelles are imperative since the microenvironment in which the protein is solubilized has physical-synthetic properties distinctive from a large volume of an aqueous solution.
For biocatalysis, the readiness of mass, naturally dissolvable permits manufactured responses to be performed by means of the control of water substance and the solubilization of hydrophobic substrates. This is proficient with a higher interfacial region (around 100 m²/mL) than the traditional biphasic frameworks, limiting mass exchange issues.
Supercells
It is a supermolecule assembly where each component comprises micelles. They are formed through different chemical approaches namely stable nanoparticles. These stable nanoparticles behave as nucleation when added to a solution from the core of the micelles or self-assembly of long cylindrical micelles that are converted into the radical cross.
Applications of Micelles
- They are widely used in electrophoresis and chromatography as a media of separation.
- They are termed as efficient favourable nano-carries in various applications namely gene delivery, diagnostic imaging, and drugs.
- Micelles act as emulsifiers when surfactants are above the critical micelle concentration allowing a compound to dissolve which is usually insoluble. For instance, detergents, and clean lipophilic materials that are less soluble which can not be removed with the help of water alone.
- They are required in the human body that plays a vital role in the removal of complex lipids and fat-soluble vitamins.

Comments