What is the Solution?
A solution is a homogeneous mixture of two or more pure substances on molecular level whose composition can vary within certain limits. A solution has two components (substances) known as solute and the solvent.
What is Supersaturated Solution?
A supersaturated solution contains more dissolved solute than required for preparing a saturated solution and can be prepared by heating a saturated solution, adding more solute, and then cooling it gently. Excess dissolved solute crystallizes by seeding supersaturated solution with a few crystals of the solute.
Table of Contents
- Recommended Videos
- Supersaturation in Phase Change
- Applications of Supersaturated Solution
- Examples of Supersaturated Solution
- Frequently Asked Questions – FAQs
Recommended Videos

For example, the object of pan boiling is the production of a fine even crop of sugar crystals. A primary condition for the attainment of this end is the maintenance of a control over the rate of crystallization during the growth. Basically, a crystal surface maintained in a solution will only grow if the concentration of the solution is maintained at a level greater than the saturation concentration. Such a solution is said to be supersaturated.
Supersaturation in Phase Change (Crystallization and Condensation)
- Physical processes and chemical processes in the vapour melt or solution phase of every system take place through the formation of three-dimensional 3D nuclei of a new phase and occur only when the medium is supersaturated.
- The formation of the nuclei is associated with a change in the free energy of the system. In the homogeneous system nuclei of the new phase are not formed as soon as the system becomes supersaturated even though thermodynamically such a situation is possible.
- The system is said to be in a state of metastable equilibrium and it can remain in that state without attaining the minimum free energy corresponding to the equilibrium state.
- In other words, in such cases nucleation of the new phase sets in after some period the value depends on such factors as the temperature and pressure of the system, the presence of chemical phases different from the nucleating phase and increased supersaturation level facilitate the process of nucleation of the new phase.
- However, there is always a supersaturation level when the new phase nucleates instantaneously. That is the new phase precipitates.
- This supersaturation level corresponds to the upper limit of the state of metastable equilibrium and defines the width of the metastable width.
Applications of Supersaturated Solution
When a solution of a solid solute dissolved in a liquid solvent is saturated, it is in thermodynamic equilibrium. In order for crystallization to occur, the state of the system must be shifted to a nonequilibrium state in which the concentration of the solute in the solution exceeds its equilibrium concentration at the given solution conditions. Solutions that are in the nonequilibrium state are said to be supersaturated. The simplest method to create a supersaturated solution is by cooling.
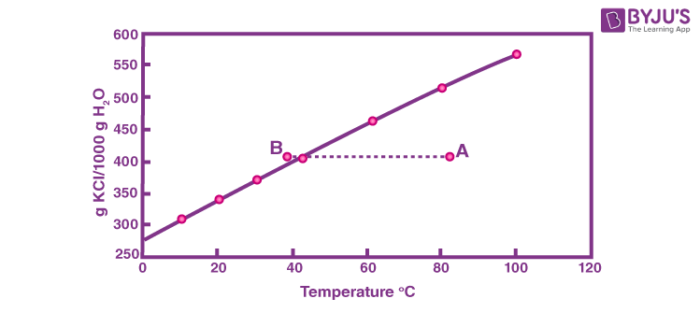
A solution is initially prepared at point A. If this solution is cooled it will be saturated when it intersects the saturation line. If it is cooled past the saturation line to point B it will be supersaturated. Just because this solution is supersaturated, however, does not mean that it will immediately crystallize. Supersaturated solutions are metastable. This means that there is a free energy barrier which must be overcome for the phase transition to be overcome.
The simplest and most common method of making a supersaturated solution is by cooling, but this is not the only method that can be used, there are many methods like solvent evaporation, temperature change, change in pH, chemical reaction and alteration in solvent composition.
Examples of Supersaturated Solution
Supersaturated solution contains more dissolved substances than a saturated solution. For example, 40g NaCl in 100ml H2O. The additional 4.0g NaCl remains undissolved.
Solved Examples
1. What is the mass percent of sodium hydroxide in a solution that is made by dissolving 8.00g NaOH in 50.0g H2O?
Solution:
Knowns 8.00g NaOH
50.0g H2O
Solving for mass percent
= 8.00g NaOH/8.00g NaOH + 50.0g H2O
= 13.8% NaOH solution.
2. Will a solution made by adding 2.5g of CuSO4 to 10g of H2O be saturated for unsaturated at 20oC?
Solution:
We first need to know the solution of CuSO4 at 20oC. From figure 14.4 we see that the solubility of CuSO4 at 20oC is about 21g per 100g of H2O. This amount is equivalent to 2.1g of CuSO4 per 10g of H2O.
Since 2.5g per 10g of H2O is greater than 2.1g per 10g of H2O, the solution will be saturated and 0.4g of CuSO4 will be maintained.
Frequently Asked Questions – FAQs
What is an example of a supersaturated solution?
A supersaturated solution is a more solute solution than can be dissolved by the solvent. If you haven’t learned what a solute / solvent is, the material that is dissolved in the solution, such as salts but not restricted to salts, is a solution. The most popular example is sodium acetate which is supersaturated.
What causes a supersaturated solution?
A supersaturated solution is a solution that contains more than the average solvent that can be dissolved at a given temperature. The recrystallization of the excess dissolved solvent in a super-saturated solution can be started by inserting a tiny solute crystal, called a seed crystal.
What is a supersaturated sugar solution?
According to the solubility of the substance a “supersaturated” solution produces more dissolved content than it should. In the case of sugar, whose chemical name is “sucrose,” approximately 211 grams of water can dissolve in 100 millilitres. Solubility is temperature dependent; more sugar dissolves in hot water than in cold.
Why are supersaturated solutions unstable?
Under appropriate conditions, solutions may often be formulated that contain a greater amount of solvent than the one required to form a saturated solution. Owing to the presence of the solute in a supersaturated solution in a concentration greater than the concentration of equilibrium, super-saturated solutions are unstable.
What happens when a supersaturated solution is cooled?
The solid crystals in the hydrated crystals will dissolve into the bath, forming a supersaturated solution. When the solution for sodium thiosulfate is gradually cooled the super-saturated solution should remain liquid. Placing a small crystal in the over-saturated solution would make the liquid solid.


Comments