Zone refining is a very useful method to get metals with high purity such as silicon and germanium. It is also referred to as zone melting, floating zone process, and travelling melting zone.
What is zone refining?
Zone refining refers to the method of purifying a crystal wherein a thin region of the crystal undergoes melting. This ‘molten zone’ is now moved across the crystal.
The impurities in the metal are melted at the forward edge by the molten zone and move through the block of metal, leaving the solidified pure element behind.
As they move through the block of metal, the impurities in the metal are concentrated in the melt and are transported to one end of the metal block. An illustration of such a process is provided below.
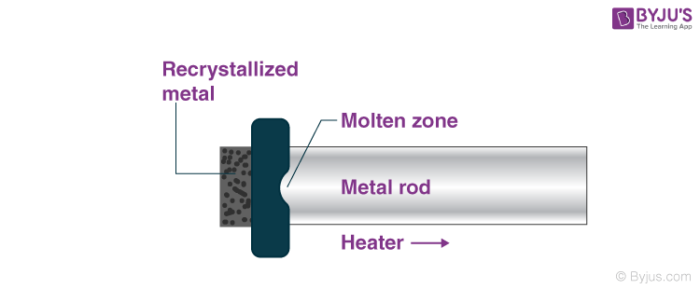
Principle of zone refining
The principle of zone refining is that the impurities in an ingot or ore of metal are more soluble in the melt state when compared to the corresponding solid-state of the impurities.
In the zone refining process, the impurities are concentrated at one end of the block of metal so that the rest of the block is purified. It can be noted that the segregation coefficient (which is defined as the ratio of impurity in the solid-state to the impurity in the liquid or melt state) is generally less than 1.
This implies that when the conditions are set at the solid-liquid boundary, the atoms of the impurity tend to diffuse into the liquid region.
Zone refining process
In the zone refining process, a circular mobile heater is fixed at one end of the metal rod which is made up of impure metal. Now, the circular mobile heater is moved slowly across the metal rod.
The metallic impurities melt at the temporary position of this heater. The melt containing the impurities moves forward along with the heater through the entirety of the metal rod. The pure metal is left to solidify as the heater moves along the rod.
As the heater moves forward, the concentration of the impurities in the melt increases. This is because the impurities are more soluble in their corresponding melt state. Finally, the impurities are accumulated at one end of the metal rod.
The process described above is repeated many times in the same direction. The end of the rod in which the impurities have now accumulated is cut off, leaving behind the pure metal. An illustration of this process is provided below.
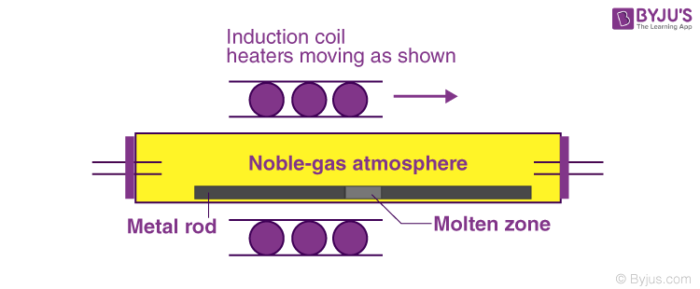
Thus, the required to be purified form of the metal is obtained. This process is very effective in the removal of impurities from semiconducting elements such as Germanium, Gallium, and Silicon. This process is also used in refining high-purity metals..

Comments