What is Atomic Size?
Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell.
In basic chemistry, the atomic radius is defined as the shortest distance between the atom’s nuclei and the outermost shell of the atom.
Table of Contents
- What is Atomic Radius?
- Recommended Videos
- Trends in the Periodic Table
- Frequently Asked Questions – FAQs
What is Atomic Radius?
An atomic radius is half the distance between adjacent atoms of the same element in a molecule.
Measuring the atomic radii of chemical elements is a complicated task as the size of an atom is of the order of 1.2×10-10 m. The electron cloud forming the shell of an atom does not have any fixed shape which makes it difficult to determine the atomic size of an atom. So we can say that practically we cannot determine the size of an individual atom.
Recommended Videos

Trends in the Periodic Table
Moving down a group or across a column or row in the modern periodic table, we can observe a lot of trends in the properties (physical and chemical) of elements in basic chemistry. For example: When we move down a group of non-metals, the reactivity of the elements decreases while it increases with moving down the group in case of representative metals.
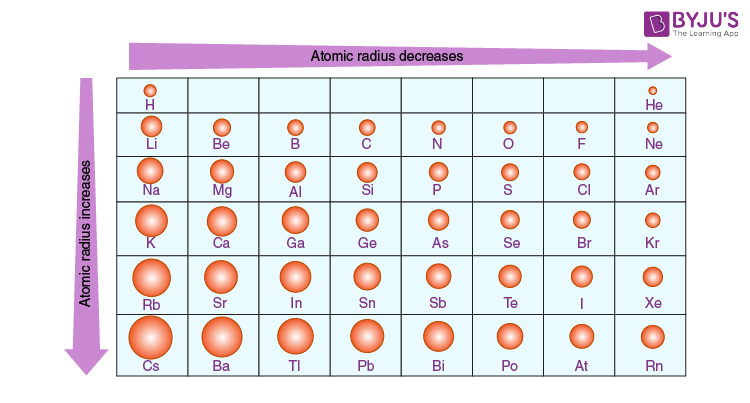
Atomic Radius Trends in the Periodic Table
When two atoms are combined, then we can estimate their atomic size by checking the distance between the atoms. The other method by which we can measure the atomic size of a non-metallic element is by forming a single covalent bond between two atoms and checking the distance between the two atoms. The radius found by this method is known as the covalent radii of the element. In the case of metal, it is termed as a metallic radius. It is defined as half of the total distance between the nuclei of two adjoining metal ions joined by a metallic bond.
The Atomic radius of an atom is measured by X-ray or other spectroscopy methods. The atomic radii of elements vary in the periodic table in a fixed pattern. We can explain this trend by considering the nuclear charge and energy level.
In general, the atomic radius decreases as we move from left to right in a period and it increases when we go down a group. This is because in periods the valence electrons are in the same outermost shell. The atomic number increases within the same period while moving from left to right which in turn increases the effective nuclear charge. The increase in attractive forces reduces the atomic radius of elements.
It was interesting to see how the force of attraction between electrons and protons plays a major role in increasing or decreasing the atomic radius.
Frequently Asked Questions – FAQs
Is atomic size and atomic radius the same?
The distance between an atom’s nucleus and its outermost shell is measured in atomic size. The atomic radius is defined as the shortest distance between the nuclei of an atom and the atom’s outermost shell in basic chemistry.
How does atomic radius affect size?
The radius of an atom grows in proportion to its atomic number. The radius of each subsequent nearby atom rises as you proceed straight down a given column on the periodic table. As you advance down the periodic table, the number of full electron shells increases, resulting in a larger size.
What increases atomic radius?
In general, atomic radius reduces as one progresses through a period and increases as one progresses through a group. The number of energy levels (n) grows as one moves down a group, resulting in a greater distance between the nucleus and the outermost orbital. As a result, the atomic radius increases.
Do neutrons affect atomic radius?
As a result, neutrons have no charge, and adding them to an atom changes its atomic mass. When neutrons are added to the nucleus, however, the nuclear radius changes.
Who discovered the atomic radius?
Radioactivity piqued Ernest Rutherford’s interest. He spent twenty years researching it, first at McGill University in Canada and then at the University of Manchester, before returning to the Cavendish as Professor in 1919.
To know more about the periodic trends in the properties of elements. Please visit BYJU’S.

Comments