What is Atomic Chemistry?
An atom is the smallest unit of ordinary matter that forms a chemical element.
Atoms are made of fundamental particles called protons, neutrons, and electrons. Protons and neutrons clump together to form a central nucleus. The electrons move in a cloud-like region around the nucleus. Most atoms are stable. Their protons, neutrons, and electrons balance.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force.
Some atoms have too many neutrons in the nucleus, which makes them unstable. They’re radioactive, giving off particles until they become stable. Atoms with extra or missing electrons are called ions. They have a positive or negative electric charge and are responsible for many chemical reactions.
Table of Contents
- Atomic Theory
- Atomic Structure
- Atomic symbol
- Atomic number
- Atomic mass
- Different atomic species
- FAQs
Atomic Theory
Dalton’s Atomic Theory
According to the postulates proposed by Dalton, the atomic structure comprised atoms, the smallest particle responsible for the chemical reactions to occur.
Postulates of Dalton’s Atomic Theory
The following are the postulates of his theory:
- Every matter is made up of atoms.
- Atoms are indivisible.
- Specific elements have only one type of atom in them.
- Each atom has its own constant mass that varies from element to element..
- Atoms can neither be created nor be destroyed but can be transformed from one form to another.
Dalton’s atomic theory successfully explained the Laws of chemical reactions, namely, the Law of conservation of mass, Law of constant properties, Law of multiple proportions, and Law of reciprocal proportions.
Discovery of subatomic particles electrons, protons, and neutrons discarded the indivisible nature of the atom proposed by John Dalton.
Atomic Structure
From the several experiments scientists proved that an atom was not the smallest indivisible particle but had a complex structure of its own and is made up of smaller particles like electrons, protons, and neutrons, etc. These particles are termed as subatomic particles.
| Subatomic particle | Charge of subatomic particle | Discovered |
| Electron | Negative (e) | J.J Thomson |
| Proton | Positive (p) | Ernest Rutherford |
| Neutron | No charge (n) | James Chadwick |
To explain the distribution of various subatomic particles inside the atom several models were proposed. These were called atomic models.
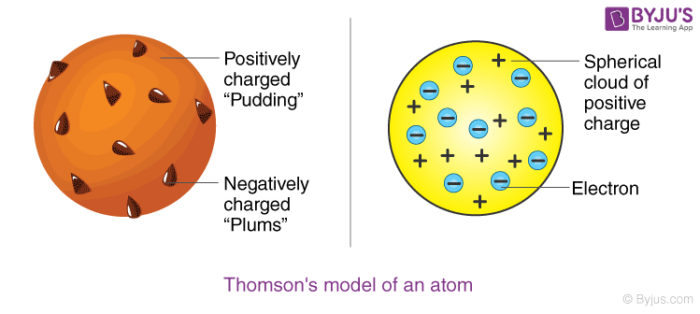
Thomson Model of Atom
It was proposed by JJ Thomson, in 1898. According to this model, an atom possesses a spherical shape in which the positive charge is uniformly distributed.
This model can be visualized as a pudding with plums or watermelon of positive charge with seeds (electrons) embedded into it. Hence, it is also called plum pudding, raisin pudding, or watermelon model.
Limitations of Thomson Model
Although this model was able to explain the overall neutrality of the atom, it was not consistent with the results of later experiments, like the α-ray scattering experiment.
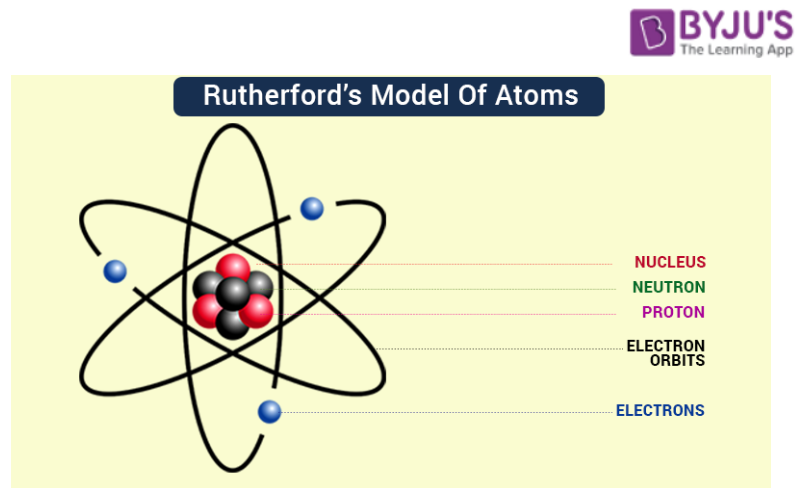
Rutherford Model of Atom
- The positively charged particles and most of the mass of an atom were concentrated in an extremely small volume called the nucleus. Nucleus is made up of nucleons.( neutrons + protons)
- There is an empty space around the nucleus called the extranuclear part, in this part electrons are present. Electrons revolve around the nucleus in closed orbits with high speed. The centrifugal force acting on the revolving electrons is being counterbalanced by the force of attraction between the electrons and the nucleus.
Limitations of Rutherford Model
According to classical electromagnetic theory, accelerated charged particles emit electromagnetic radiation and hence an electron revolving around the nucleus should emit electromagnetic radiation. This radiation would carry energy from the motion of the electron which would come at the cost of shrinking of orbits. Ultimately the electrons would collapse in the nucleus. So Rutherford model was not in accordance with classical electromagnetic and could not explain the stability of an atom.
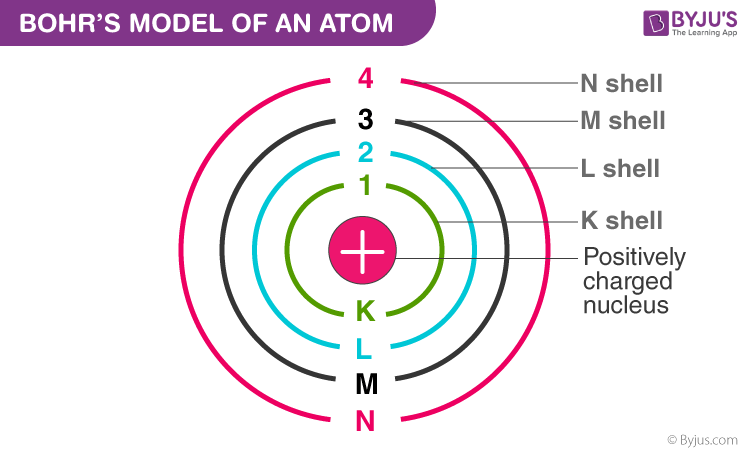
Bohr’s Model of Atom
Postulates of Bohr’s model are:
- Electrons revolve around the nucleus in a specific circular path known as orbital, energy of each orbital is constant.
- Energy absorbed or emitted during the transition is given by ΔE = hν
- The angular momentum of an electron present in any orbital must be an integral multiple of h/2π
Limitations of Bohr’s Model
- Bohr’s model was not able to explain the Heisenberg uncertainty principle.
- It failed to explain the Stark effect (effect of electric field on the spectra of atoms).
- It is also failed to explain the Zeeman Effect (effect of magnetic field on the spectra of atoms)
Recommended Video
Atomic Models – Structure of Atom

Atomic Symbol
Atom of an element is represented in the form of an atomic symbol.
| Element | Atomic symbol |
| Hydrogen | H |
| Helium | He |
| Lithium | Li |
| Beryllium | Be |
| Boron | B |
To know the atomic symbol of 118 element you may visit ➡ Atomic symbol
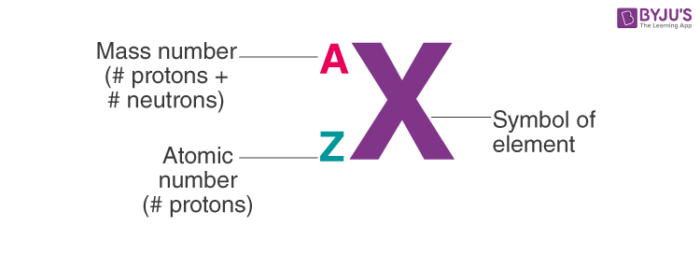

Atomic Number (z)
The number of protons present in the nucleus of an atom is equal to its atomic number (Z).
Thus Atomic number (Z) = number of protons in the nucleus of an atom
Example Atomic number of Carbon(C) = 6 = number of protons
To know the atomic number of 118 elements you may visit ➡ Atomic number
Atomic Mass (A)
Atomic mass is equal to the sum of protons and neutrons present in the nucleus of the atom.
Thus Atomic mass = number of protons + number of neutrons
For Example Atomic mass of carbon = 12
To know the atomic mass of element you may visit ➡ Atomic mass
Different Atomic Species
The types of atomic species are:
Isotopes
Atoms of the same element which have the same atomic number (or number of protons) but have different mass numbers (or number of neutrons) are called isotopes.
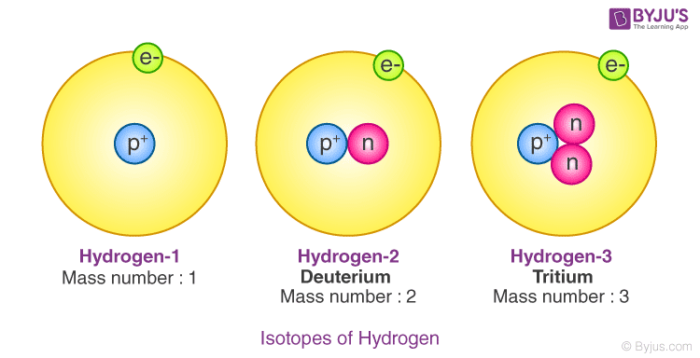
To learn more about its properties you may visit Isotopes of elements
Isobars
Atoms of different elements that have the same mass number but different atomic numbers are called Isobars.
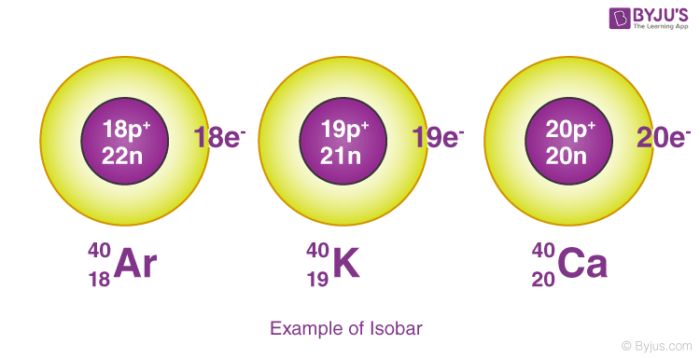
To learn more about the difference between isotopes and isobars visit Isotopes and isobar
Frequently Asked Questions on Atomic
Who discovered atoms?
The idea that everything is made of atoms was pioneered by John Dalton.
What is an atom made of?
Atoms are made of fundamental particles called protons, neutrons, and electrons. Protons and neutrons clump together to form a central nucleus. The electrons move in a cloud-like region around the nucleus.
Are atoms particles or waves?
All matter, which appears to be particulate, can also behave like waves. Atoms are both particles or waves.
Can an element be one atom?
The simplest structural unit of an element is an atom. An element is a substance that is made entirely from one type of atom.
What are atomic mass and atomic number?
Atomic mass is equal to the sum of protons and neutrons present in the nucleus of the atom. The number of protons present in the nucleus of an atom is equal to its atomic number.
Comments