What is Ammonium Nitrate?
Ammonium nitrate is an ionic salt made up of the ammonium cation (NH4)+ and the nitrate anion (NO3)–. The chemical formula of this compound is NH4NO3.
Since Ammonium Nitrate contains half of the nitrogen (N) in the nitrate form and half in the ammonium form, ammonium nitrate is a widely used fertiliser. The nitrate form is easily transported to the roots with the help of soil moisture and is thus instantly available for plant absorption.
Table of Contents
- Structure of Ammonium Nitrate – NH4NO3
- Preparation of Ammonium Nitrate – NH4NO3
- Properties of Ammonium Nitrate
- Uses of Ammonium Nitrate
- FAQs
Structure of Ammonium Nitrate
This compound features an ionic bond between an ammonium ion and a nitrate ion. The structure of an NH4NO3 molecule is illustrated below.
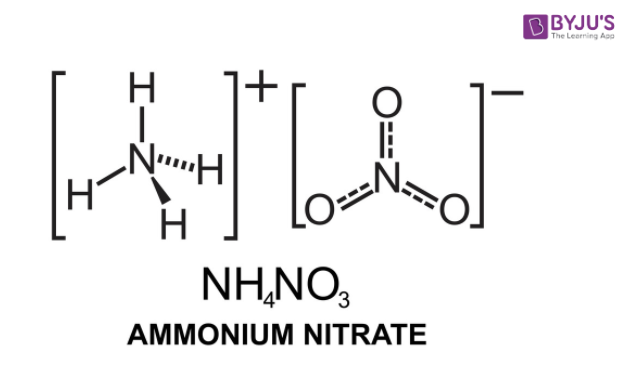
The pi electrons in the nitrate ion are delocalized due to resonance. The net charge on this ion is -1 (since the nitrogen atom holds a charge of +1 and each oxygen atom holds a charge of -⅔). Therefore, only one NH4+ ion can form an ionic bond with one NO3– ion.
Chemical Data
| Chemical Formula | NH4NO3 |
| Molar Mass/ Molecular Weight | 80.043 grams per mole |
| Density | 1.725 grams per cubic centimetre |
| Melting Point | 442.8K (169.6oC) |
| Boiling Point | Decomposes at 483K (210oC) |
Preparation of Ammonium Nitrate
Ammonium nitrate readily dissolves in water by dissociating into its constituent ions. This salt is acidic in nature since it is derived from a weak base (NH3) and a strong acid (HNO3).

NH4NO3 can be prepared from the acid-base reaction between nitric acid and ammonia, described by the following chemical equation:
NH3 + HNO3 → NH4NO3
This reaction is highly exothermic and proceeds violently. Ammonium nitrate is also known for its oxidizing powers. It is widely used in many explosives used in the mining and construction sectors. NH4NO3 is also one of the key components of ANFO, one of the most popular choices of industrial explosives.
Ammonium Nitrate Properties
Physical Properties
- Ammonium nitrate is a crystalline solid having a white/grey colour.
- It has a trigonal crystal structure.
- It is quite soluble in water; its solubility at 20oC is 150g/100ml. The solubility increases to 1024g/100ml when the temperature is raised to 100o
- The dissolution of NH4NO3 in H2O is highly endothermic.
- This compound has very low shock and friction sensitivities.
Chemical Properties
- When NH4NO3 is reacted with the hydroxides of alkali metals, the nitrates of alkali metals are formed along with ammonia.
- Upon heating, this compound decomposes to form nitrous oxide (N2O) and water.
- When exploded, this compound yields N2, O2, and water as the byproducts.
- Despite being a component of many explosives, ammonium nitrate is not an explosive by itself. It must be mixed with a primary explosive such as an azide to form an explosive mixture.
Ammonium Nitrate as Fertilizer
Since nitrogen is an essential component of both the NH4 (ammonium) and NO3 (nitrate) components of the molecule, ammonium nitrate is a widely used fertiliser. In addition to plants being able to absorb nitrogen directly from the molecule in its nitrate form, soil microorganisms can also progressively change the ammonium part of the complex into nitrate. Due to these advantages, ammonium nitrate is a preferred nitrate source for plant nutrition by many vegetable farmers. Ammonium nitrate is used by animal farmers for fertilising pasture and hay since urea-based fertilisers have a tendency to volatilize from the soil before the plants can absorb them. Additionally, it is very soluble, making it a good choice for irrigation systems.
Uses of Ammonium Nitrate
Some important applications of this ionic salt are listed below.
- NH4NO3 is a key component of many fertilizers since it is quite rich in nitrogen (34%).
- Another advantage of this compound is that it doesn’t lose its nitrogen to the atmosphere, as is the case with urea.
- When mixed with fuel oil, it forms an explosive known as ANFO, which is one of the most popular industrial explosives.
- The dissolution of this compound in water is quite endothermic, making it an ideal choice of material in instant cold packs.
- It is also used in the mining and construction industry as a component of explosives.
Despite its numerous applications, the use of this compound is being slowly phased out by the governments of many countries due to its scope for misuse. Ammonium nitrate was one of the key ingredients of the explosives that were used in the 2011 Delhi bombings and the 2013 blasts in Hyderabad.
Frequently Asked Questions – FAQs
What is ammonium nitrate used for?
Used to make fertilizers and explosives, and as a nutrient in antibiotic and yeast growth. Ammonium nitrate is the nitric acid ammonium salt. It has a function as a fertilizer, an oxidizing agent and an explosive.
Is ammonium nitrate a base or acid?
Ammonium nitrate is not acid but a salt but the solution is acidic since it is a salt with a weak base (ammonium hydroxide) and a heavy nitric acid.
What happens when you mix water and ammonium nitrate?
It feels cold when ammonium nitrate is dissolved in water which indicates an endothermic reaction. In an endothermic reaction, the ammonium nitrate dissolves in water, a chemical reaction that absorbs heat rather than releases it. Ammonium nitrate consists of tightly packed, ionic bonds.
What can you do with ammonium nitrate?
Ammonium nitrate is a chemical compound used as a fertilizer in agriculture, for producing pyrotechnics, as an ingredient in cold packs, and for scientific demonstrations. This is also used in mining and quarrying to produce controlled explosions.
Is ammonium nitrate a good fertilizer?
Ammonium nitrate fertilizer is the compound’s most common application but it also has a very volatile quality, making it useful in some industries. The use of ammonium nitrate in gardens and large-scale growing fields stimulates plant growth and provides a ready source of nitrogen that plants can draw from.
To learn about other ionic compounds of the nitrate anion, such as potassium nitrate, register with BYJU’S and download the mobile application on your smartphone.

Comments