Table of Contents
What is Butanoic acid?
Butanoic acid is an oily colourless liquid with the chemical formula C4H8O2. It is a short chain saturated fatty acid found in the form of esters in animal fats and plant oils. It was discovered by Lieben and Rossi in 1869. It is also called butyric acid which means the acid of butter as it was first discovered in rancid butter. It was prepared by the butyric fermentation of carbohydrates and by the oxidation of n-butyl alcohol.
Other names – butyric acid, n-Butyric acid, n-Butanoic acid
| C4H8O2 | Butanoic acid |
| Density | 960 kg/m³ |
| Molecular Weight/ Molar Mass | 88.11 g/mol |
| Boiling Point | 163.5 °C |
| Melting Point | -7.9 °C |
| Chemical Formula | CH3CH2CH2-COOH |
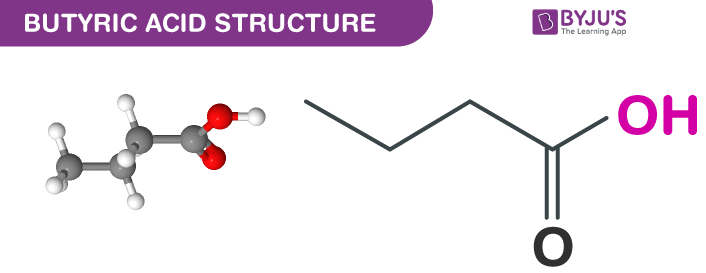
Butanoic acid Structure – C4H8O2
Physical Properties of Butanoic acid – C4H8O2
| Odour | Unpleasant odour |
| Appearance | Colourless clear liquid |
| Covalently-Bonded Unit | 1 |
| Heat capacity | 298.15 K, 1 atm |
| Complexity | 49.5 |
| Solubility | Miscible in water and ethanol. |
Chemical Properties of Butanoic acid – C4H8O2
-
-
-
-
- Butanoic acid reacts with sodium hydroxide to form the sodium salt of butanoic acid and carbon dioxide and water.
-
-
-
21C4H8O2 + 20NaOH → 20 NaC4H6O + 4CO2 + 34H2O
-
-
-
-
- Butanoic acid on treatment with water forms acetic acid and ether. The chemical equation is given below.
-
-
-
C4H8O2 + H2O → CH3COOH + C2H6O
Uses of Butanoic acid – C4H8O2
-
-
-
- Used in the manufacture of esters for artificial flavourings, as a food additive, in the manufacture of varnishes, and in decalcifying hides.
- Used in the manufacture of perfume, flavourings, pharmaceuticals, and disinfectants.
- Used as an important flavouring agent in a number of food, including beer and may be present in cosmetic and detergent preparation.
- It is recognized as a safe food additive when used in accordance with good manufacturing practices or feeding practices.
-
-
Frequently Asked Questions
What are the uses of butanoic acid?
Butanoic acid, also known as butyric acid, is used to prepare specific esters of butyrates. This compound is also used to manufacture butyrate of cellulose acetate (CAB), which is used in a wide range of devices, components, and coatings, and is more resistant to degradation than the acetate of cellulose.
How is butanoic acid prepared?
Butyric acid is industrially prepared by Butyraldehyde oxidation. Saturation with salts such as calcium chloride can isolate it from the aqueous solutions. When dissolved in hot water, the calcium salt, Ca(C4H7O2)2·H2O, is relatively less soluble.
Comment on the microbial biosynthesis of butanoic acid
Butyrate is generated by multiple fermentation processes performed by obligatory anaerobic bacteria. Louis Pasteur discovered this fermentation pathway in 1861. Examples of bacteria which produce butyrate include Clostridium butyricum, Clostridium kluyveri and Clostridium pasteurianum

Comments