What is Carbon Tetrachloride?
Carbon tetrachloride, also known as tetrachloromethane, is an organic compound with the chemical formula CCl4. This compound is often classified as a polyhalogenated organic compound since it consists of a carbon atom which is attached to more than one halide functional group. Under standard conditions for temperature and pressure, CCl4 exists as a colourless liquid which emanates a very sweet odour.
In the past, this compound was widely used in cleaning agents. It was also used in fire extinguishers and was known to serve as a precursor to several refrigerants. However, the use of this compound has been phased out by several governments due to its toxicity. The inhalation of large quantities of carbon tetrachloride can cause serious damage to vital organs like the kidney and liver. It can also have negative effects on the central nervous system (CNS). Furthermore, prolonged human exposure to carbon tetrachloride often results in death.
Carbon tetrachloride was also widely used as a precursor to chlorofluorocarbons (CFCs). However, due to environmental concerns, the production of this compound has seen a sharp decline since the 1980s. Exposure to carbon tetrachloride is known to cause centrilobular hepatic necrosis. In the body, this compound is metabolized into the trichloromethyl radical, which is highly reactive. This trichloromethyl can go on to cause hepatocellular damage.
Table of Contents
- Structure of CCl4 Molecules
- Properties of Carbon Tetrachloride
- Key Applications of Tetrachloromethane
- Health Hazards Associated with CCl4
- Related Videos
- Frequently Asked Questions on Carbon Tetrachloride
Structure of CCl4 Molecules
Carbon tetrachloride molecules have tetrahedral molecular geometries in which the central carbon atom is bonded to four chlorine atoms. The structure of CCl4 molecules is illustrated below.
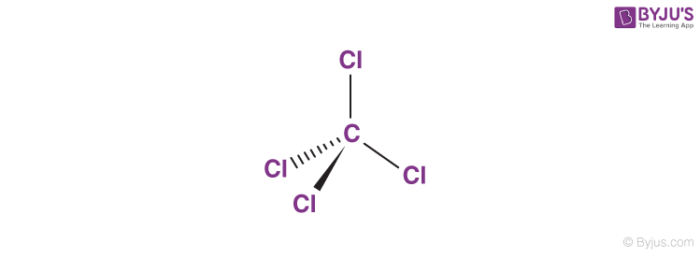
It can be noted that the four chlorine atoms are positioned symmetrically at each corner around the central carbon atom in the CCl4 molecule. The bonds between the carbon atom and the chlorine atoms are covalent. As a consequence of the molecular geometry of this compound, it exhibits non-polar properties. It can be noted that the molecular structure of CCl4 is quite similar to that of CH4 (methane gas).
Properties of Carbon Tetrachloride
- Some important physical and chemical properties of CCl4 are listed below.
- The molar mass of carbon tetrachloride is 153.81 grams per mole.
- Under standard conditions, this compound exists in the liquid state. It has a colourless appearance and a sweet odour.
- The density of this compound (in its liquid state) corresponds to 1.5867 grams per cubic centimetre.
- The melting point of carbon tetrachloride is -22.93oC whereas the boiling point of this compound corresponds to 76.72oC.
- CCl4 is not very soluble in water. At a temperature of 25 degrees Celsius, the solubility of carbon tetrachloride in water is only 1 gram per litre (approx.). However, it can be noted that this compound is soluble in many organic solvents such as chloroform, benzene, alcohols, ethers, and formic acid.
- Carbon tetrachloride crystallizes in a monoclinic crystal lattice.
- The coordination geometry of this compound has a tetrahedral shape. The molecular shape of this compound is also tetrahedral. It can be noted that the central atom in this scenario is the carbon atom.
- The thermal capacity or the heat capacity of carbon tetrachloride is 132.6 Joules per mole Kelvin.
- The standard molar entropy associated with this organic compound is 214.42 Joules per mole Kelvin.
Key Applications of Tetrachloromethane
Before the 1980s, the primary application of carbon tetrachloride was in the production of chlorofluorocarbons for refrigeration. It was also used as a component of fire extinguishers and as a cleaning agent. However, despite the health hazards associated with this compound and the serious environmental damage caused by chlorofluorocarbons (see: ozone layer depletion), the use of this compound has been phased out by the governments of several countries. However, this compound is known to have other niche uses, some of which are listed below.
- Carbon tetrachloride is used as a chlorine source in a named reaction known as the Appel reaction.
- It is also used to reveal the watermarks that are placed on stamps without causing any damage to the stamp in the process.
- Carbon tetrachloride was also used as a component in the manufacture of lava lamps.
- Historically, CCl4 has been used in proton NMR spectroscopy.
Health Hazards Associated with CCl4
Carbon tetrachloride is a highly potent hepatotoxin which can cause serious damage to the liver. In high enough concentrations, this compound can also damage the central nervous system (CNS). Exposure to CCl4 for prolonged durations of time often leads to death or coma. Exposure to this compound has also been linked to cancer and kidney damage.
Related Videos
Frequently Asked Questions on Carbon Tetrachloride
What are the uses of carbon tetrachloride?
What happens when carbon tetrachloride is exposed to steam?
Comment on the structure of carbon tetrachloride.
Why is carbon tetrachloride considered to be dangerous?
What does carbon tetrachloride smell like?
To learn more about this topic and other related topics, such as the effects of dichloromethane on the environment, download BYJU’S – The Learning App.

Comments