Do you know how coal and petroleum were formed?
Coal and petroleum mainly consist of carbon and its compounds. They are the most used form of fossil fuels. Coal is formed when the dead organic matter such as plants and animals decay and are subjected to high pressure and temperature for a very long period of time under the earth’s surface. The formation of coal and petroleum is a continuous process and it takes millions of years for the formation of these fossil fuels by natural processes. Since the major content of coal is carbon and the formation of coal starts with dead organic matter, the process of conversion of dead organic matter into coal is referred to as carbonization.
Table of Contents
- Oxidations reactions of carbon and its compounds
- Addition reactions of carbon and its compounds
- Substitution reactions of carbon and its compounds
- Combustion reactions of carbon and its compounds
- Frequently asked questions- FAQs
Reactions associated with carbon and its compounds.
Let us know something about the reactions involved with the carbon compounds.
Oxidation reactions of carbon and its compounds:
Carbon and its compounds get easily oxidized when subjected to combustion. For example alcohol on oxidation is converted to carboxylic acid. The substances which can add oxygen to other compounds are known as oxidising agents. Some examples of oxidising agents include alkaline potassium permanganate and potassium dichromate. Carbon burns in the presence of oxygen and gets oxidized to carbon dioxide and carbon monoxide depending upon the availability of oxygen and the extent of reaction. Most compounds of carbon get oxidized when reacted with strong oxidizing agents.
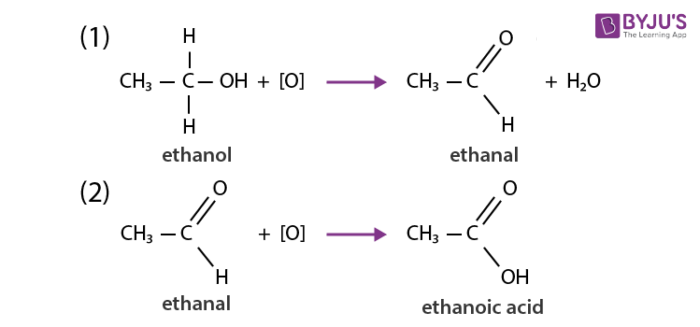
Addition reactions of carbon and its compounds:
Unsaturated hydrocarbons react with hydrogen in the presence of catalysts to give saturated hydrocarbons and this reaction is known as addition reaction. A catalyst is a substance that enhances the rate of a chemical reaction without itself getting changed. Addition reaction is observed only for those compounds that have unsaturation that is the compounds that contain multiple bonds (double bond, triple bond) in them. Industrially, hydrogenation of vegetable oils is an example of addition reaction in which hydrogen is added to unsaturated vegetable oil in presence of a catalyst to convert it into a saturated compound.

Also read : Addition Reactions
Substitution reaction of carbon and its compounds:
The reactions in which a less reactive element or molecule is replaced by a more reactive element or molecule are known as substitution reactions. Substitution reaction is mainly observed in case of saturated hydrocarbons. The reaction of saturated hydrocarbons in presence of sunlight with chlorine is an example of substitution reaction. Chlorine being more reactive has the ability to displace the hydrogen atom from the saturated hydrocarbons. Here the products formed are usually higher homologues of the same hydrocarbon.
To know more about carbon and its compounds visit byjus.com visit : carbon and its compound
Combustion reaction of carbon and its compounds:
As we know that most of the fuel is made up of carbon and its compounds, let us study about the reaction that releases energy from these compounds, combustion
When carbon burns in the presence of oxygen, it produces heat and light. This process of burning carbon and its compounds to release energy is known as combustion. Combustion of fuels are widely used across the various industries for the extraction of energy from them. This energy is then used to operate different equipment, machineries, cooking etc.

Examples
i)
ii)
The colour of the flame of combustion depends on the carbon compound being used. We will get clean flame with saturated hydrocarbons while unsaturated carbon compounds will produce yellow flame with black smoke. Ultimately, the burning of carbon compounds results in a sooty deposit on the surface. If there is an incomplete combustion of saturated hydrocarbons due to limited supply of air, a sooty flame will be observed. You must have observed in your home that kerosene stove has inlets for the passage of air so that a mixture of oxygen and kerosene is burnt to give a clean blue flame. We also observe sometimes that the bottom of the vessel is blackened which means the passage has been blocked and the fuel is almost wasted. One of the major air pollutants are the oxides of sulphur and nitrogen which are mainly produced after the combustion of coal and petroleum fuels.
Have you ever thought why do some substances burn with flame and some without flame?
Observe the difference when we burn coal/wood and when we burn candle. You will see that candle burns with a flame and gives both heat and light but in case of coal/wood you will observe that they just gives a red glow and do not produce any flame. This is because, a flame is observed only when a gaseous substance is burnt.
The colour of the flame depends upon the characteristic properties of the material. To know about other reactions involved in carbon compounds log on to www.byjus.com.’
Frequently Asked Questions- FAQs
1. What are the four chemical properties of carbon?
Carbon mainly takes part in the four main types of reaction such as Oxidation reactions, Addition reactions, Substitution reactions and Combustion reactions. Carbon burns in the presence of oxygen, it produces heat and light.
2. What is the chemical property of carbon dioxide?
Carbon dioxide is neither combustible nor a supporter of computation. It burns with metals like sodium, potassium, and magnesium . An aqueous solution of carbon dioxide is weakly acidic due to the formation of carbonic acid.
3. What are the characteristics of carbon?
Carbon shows a maximum covalence of four in its compound and doesn’t take part in complex formation. It is highly unreactive under normal conditions. Carbon relative size is quite small due to which it’s compounds are quite stable under normal conditions.
4. What are the unique properties of carbon?
Carbon has a maximum tendency to catenate. It participates in multiple bonding with carbon, oxygen and nitrogen.
5. What are allotropes of carbon?
Elemental carbon exists in a number of allotropic forms which are both crystalline and amorphous in nature. A few important allotropic forms of carbon are graphite, diamond , fullerene etc.


Comments