Electromagnetic Radiation is basically light, which is present in a rainbow or a double rainbow. It also is a spectrum consisting of radio waves, microwaves, infrared waves, visible light, ultraviolet radiation, X-rays, and gamma rays. There are only two ways to transfer energy from one place to another place.
- Through a particle.
- Through a wave.
Electromagnetic radiation is a particle as well as a wave thus it is interesting to study its nature in quantum theory. Light is also an electromagnetic radiation which contains frequencies.
Dual Behaviour of Electromagnetic Radiation
The photoelectric effect could be explained considering that radiations consist of small packets of energy called quanta. These packets of energy can be treated as particles. On the other hand, radiations exhibit a phenomenon of interference and diffraction which indicated that they possess a wave nature. So it may be concluded that electromagnetic radiations possess dual nature.
- Particle nature
- Wave nature
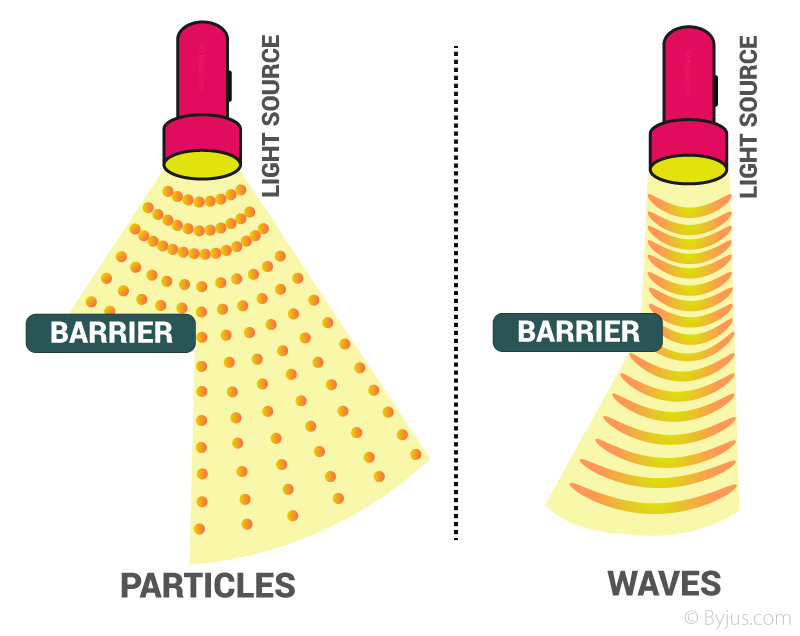
Particle Nature of Electromagnetic Radiation
Planck’s Quantum Theory – It states, “One photon of light carries exactly one quantum of energy.” Wave theory of radiation cannot explain the phenomena of the photoelectric effect and black body radiation.
What is Black Body Radiation?
An ideal black body is a perfect absorber and perfect emitter of radiation. When such a body is heated, it becomes red-hot. In other words, it emits red coloured light. As the temperature is further increased, the colour of the radiation emitted changes from red to yellow to white and finally purple as the temperature becomes very high. This implies that the wavelength of radiation emitted by the black body decreases with an increase in temperature.
Wave Nature of Electromagnetic Radiation
Due to a dispersion of white light, VIBGYOR appears. Vibgyor stands for – Violet, Indigo, Blue, Green, Yellow, Orange, and Red.
Emission & Absorption Spectrum
Spectra can be divided into two types based on absorption by gas or vapour & white light emission:
- Emission Spectrum: An spectrum due to the emission of white light by gas at high temperature is known as an emission spectrum. This kind of spectrum usually consists of bright lines on a dark background. The emission of energy by electrons generates an emission spectrum.
- Absorption Spectrum: The spectrum which occurs due to absorption of white light by gas and transmitted white light, is termed an absorption spectrum. Unlike the emission spectrum, it consists of dark lines on a bright background. it is due to the absorption of energy by electrons.
Spectra can be divided into two types depending on the spectral lines:
- Line Spectrum or Atomic Spectrum – This is made up of distinct lines. When an electron in an atom excites and de-excites, this spectrum occurs. Emission & absorption spectra show the line spectrum.
- Band Spectrum – It is a characteristic of a molecule. It consists of closely spaced lines called bands. In a molecule, the vibration & rotation of atoms generates such spectrums.
Frequently Asked Questions – FAQs
What are matter waves?
Write de-Broglie equation.
λ is the de- Broglie wavelength
m is the mass
v is the velocity of the particle
Name the scientists who experimentally verified the de Broglie’s hypothesis.
Do charged particles exhibit wave properties?
To learn more and solutions for NCERT dual nature of radiation and matter, register with BYJU’S and download our app.

Comments