What is Ethanoic Acid?
Ethanoic acid (CH3COOH) belongs to the group of carboxylic acids and is commonly called as acetic acid. It is slightly heavier than water with a density of 1.05 g/cm3. After adding 5-8% of acetic acid in water it becomes vinegar and is mostly used as preservatives in pickles. Acetic acid is the common name for Ethanoic acid.
Table of Content
Structure of Ethanoic acid
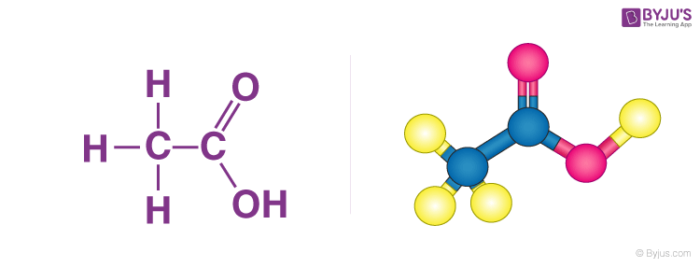
Properties of Ethanoic acid
| Chemical formula | CH3COOH |
| Molecular Weight/ Molar Mass | 60.05 g/mol |
| Density | 1.05 g/cm3 |
| Boiling Point | 118oC |
| Melting Point | 16oC |
What is glacial acetic acid?
Ethanoic acid is also referred to as glacial acetic acid because its melting point is 16oC. Hence, it often freezes in winter when the climate is cold.
Reactions of Ethanoic acids:
Esterification reaction:
- When carboxylic acid and alcohol react, the product formed is known as an ester. Below is an example of the formation of an ester from the reaction of ethanoic acid with absolute ethanol in the presence of an acid as a catalyst.
CH3COOH + CH3CH2OH → CH3COOCH2CH3
(Ethanoic acid) (Ethanol) (Esters)
- Esters have a sweet fruity smell. They are mainly used for making perfumes and synthetic flavouring agents. The reaction of esters with alkalis gives carboxylic acid salt and alcohol. This reaction is used in the making of soaps and the process is called as saponification reaction.
CH3COOC2H5 + NaOH → C2H5OH + CH3COONa
Reaction with a base:
- Ethanoic acid reacts with a base to give the salt and water just like other mineral acids.
Reaction with carbonates and hydrogen carbonates:
- Carbon dioxide, salt, and water are produced when ethanoic acid reacts with carbonates and hydrogen carbonates. Sodium acetate is usually produced as a salt when ethanoic acid reacts with sodium bicarbonate as shown in the reaction below:
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Uses of Ethanoic Acid
- Ethanoic acid is widely used in many industries.
- Commercially it is used in the manufacturing of esters, vinegar, and many polymeric materials.
- Vinegar has been shown to reduce high concentrations of blood sugar.
- Used as an agent to lyse red blood cells before white blood cells are examined.
- Used as a solvent in the production of camphor, ascent and cooking ingredient.
- Used as a stop bath for photographic emulsion development.
- Farmers sometimes spray acetic acid on livestock silage to fight fungal and bacterial growth.
Frequently Asked Questions – FAQs
What is Ethanoic acid used for?
Acetic acid is used to make metal acetates, which are used in some printing processes; vinyl acetate, which is used to make plastics; cellulose acetate, which is used to make photographic films and textiles; and volatile organic esters.
Is Ethanoic acid used to preserve food?
Acetic acid is utilised for more than just salad dressing. It is utilised as a natural preservative and antibacterial agent in the preservation of food, not only in vegetable pickling but also as an active component of edible films and in hurdle preservation technology.
Is Ethanoic acid a vinegar?
Ethanoic acid, often known as acetic acid, is simply ethanoic acid diluted in water. The majority of ethanoic acid is now synthesized and utilized in the production of other essential compounds, such as plastics.
Why is Ethanoic acid a weak acid?
Because it does not release all of its hydrogen in water, ethanoic acid (acetic acid) is classified as a weak acid. Instead, it dissociates partly and achieves equilibrium with its conjugate base.
Why do esters smell sweet?
The aroma of the ester produced by acetic acid and ethanol is sweet. – There is a modest intermolecular interaction between the esters. Ester compounds are volatile in nature due to the lower intermolecular force of attraction. – The hydrogen bond does not exist in esters as well.
Learn more about the chemical behaviour and importance of ethanoic acid with the expert faculties at BYJU’S.

It is a good work .