Class 11 chemistry important 3 mark questions with answers are provided here. These important questions are based on CBSE board curriculum and correspond to the most recent Class 11 chemistry syllabus. By practising these Class 11 important 3-mark questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 11 Annual examinations as well as other entrance exams such as NEET and JEE.
Download Class 11 Chemistry Important 3 Mark Questions with Answers PDF by clicking on the button below.
Class 11 Chemistry Important 3 Mark Questions with Answers
Q1:
Give reasons:
- LiCl is more covalent than KCl.
- In an aqueous solution Li+ has the lowest mobility.
Answer:
- The reason behind this is a phenomenon known as polarisation. It causes a partly covalent character to arise in an ionic bond. It is determined by four elements, two of which are:
- The smaller the cation, the greater the attraction and polarisation.
- The larger the anion, the greater the distortion and polarisation.
The anion is the same, Cl–, but the cation Li+ is significantly smaller than K+, causing LiCl to polarise more than KCl, making it more covalent.
Q2:
Describe in detail the expanded octet with suitable examples.
Answer:
The octet rules stipulate that when an atom’s valence shell is complete with eight electrons, it is the most stable. It is based on the observation that the atoms of main group elements tend to participate in chemical bonding in such a way that each atom in the resulting molecule has eight electrons in the valence shell. Only the core group elements are affected by the octet rule.
The octet rule is known to apply to halogen, oxygen, nitrogen, and carbon compounds. The s-block elements and the p-block elements are examples of elements that follow this rule in general (except hydrogen, helium, and lithium).
An expanded octet is made up of elements like sulphur, phosphorus, silicon, and chlorine. More than 8 electrons surround the core atom in compounds like phosphorus pentachloride (PCl5) and sulphur hexafluoride (SF6), which stray from the octet rule.
Taking NaCl (Sodium Chloride) as an example:
- The sodium ion (Na+) and the electronegative chloride ion (Cl–) form an ionic connection in this molecule.
- The chlorine atom has 7 electrons in its valence shell and can gain an electron to form an octet configuration.
- One electron exists in sodium’s outermost shell. If this electron is lost, the second shell becomes the valence shell (which is already filled with 8 electrons). As a result, the Na+ ion has higher stability than metallic sodium.
- The sodium cation and chloride anion have now formed an ionic bond, and the resulting molecule has octet configurations for both atoms.
Q3:
(a)Give the importance of measuring the BOD of a water body.
(b) What is the desired concentration of fluoride ion pH in drinking water?
(c) Calculate the pH of a buffer solution containing 0.2 moles of NH4Cl and 0.1 moles of NH4OH per litre. Given Kb for NH4OH = 1.85 X 10-5
Answer:
(a) The biological oxygen demand, or B.O.D., is a measure of pollution in water generated by organic biodegradable materials. When the B.O.D. level is low, it signifies that the water contains less organic matter.
BOD in water is a measurement of the quantity of organic material present in water in terms of how many oxygen molecules are needed to break it down biologically. The BOD value of clean water is less than 5 ppm, but the BOD value of highly contaminated water is 17 ppm or more.
(b) The maximum recommended level of fluoride in drinking water is 1.0 ppm, whereas the maximum permissible value is 1.5 ppm, according to the World Health Organisation. Fluorosis of the skeleton and teeth can occur at very high doses. Dental cavities can be prevented by having a low fluoride content of less than 0.5 ppm in the mouth.
(c) According to Henderson – Hasselbalch equation:
pOH = pKb + log ([salt]/ [base])
pKb = – log Kb
= – log (1.85 x 10-5)
= – log 1.85 + (– log 10-5)
= -0.2671 + 5
= 4.73
Therefore, pOH = 4.73 + log (0.2 / 0.1)
= 4.73 + log 2
= 4.73 + 0.301
= 5.0339
pH + pOH = 14
pH = 14 – pOH
= 14 – 5.0339 = 8.9661
Q4:
Explain the hybridisation of SF4.
Answer:
Hybridization is the process of combining atomic orbitals belonging to the same atom with slightly varying energies such that energy is redistributed between them, resulting in the development of new sets of orbitals with equivalent energies and shapes.
To figure out how sulphur tetrafluoride hybridises, we must first figure out its Lewis structure and the number of valence electrons it contains. A total of 34 valence electrons make up the SF4 molecule. Sulphur will provide six electrons, and each of the four fluorine atoms will have seven.
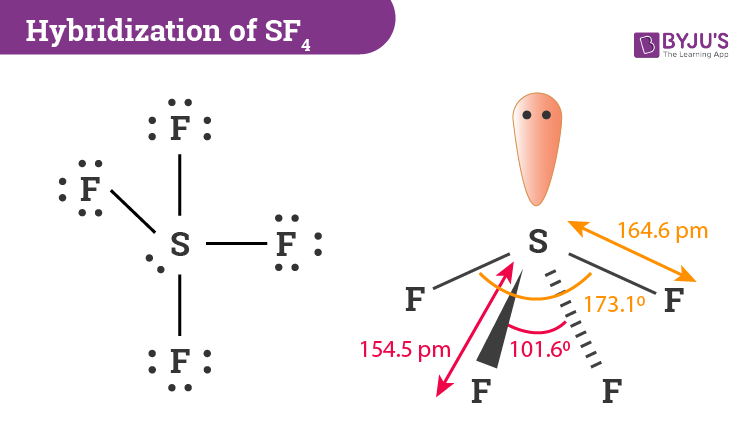
SF4 only has one lone pair and four F sigma bonds. S is the core atom. To put it another way, it has four bonding zones, each with one lone pair.
There are 5 electron pairs and 34 valence electrons. The five valence atomic orbitals on the central atom S are hybridised to produce five sp3d hybrid orbitals as a result. Four of the hybrid orbitals cross paths with 2P-orbitals. The last hybrid orbital retains a lone pair when this entire process is completed.
During the synthesis of SF4, the sulphur atom forms 8-valence electron bonds with each of the fluorine atoms. Furthermore, the four fluorine atoms will have three lone pairs of electrons in their octet, requiring a total of 24 valence electrons. A lone pair of electrons will also be placed in the sulphur atom. We can now calculate Sulphur’s hybridization by counting the number of electron density zones.
In Sulphur, bonding results in the formation of four single bonds, with only one lone pair. As a result, we can claim that there are 5 electron density areas.
The middle S atom containing the 5 valence atomic orbitals is basically hybridised to form five sp3d hybrid orbitals. Four hybrid orbitals overlap in 2P-orbitals, with the fifth comprising a lone pair. The steric number can also be used to calculate the number of hybrid orbitals used by the atom. Sulphur will have five orbitals containing one 3s-orbital, three 3p-orbitals and one 3d-orbital.
Q5:
(a) Calculate the total number of electrons present in one mole of methane.
(b) An atomic orbital has n = 3. What are the possible values of l and m1?
Answer:
(a) The solution is given below:
1 molecule of methane contains 6 Carbon electrons and 4 Hydrogen electrons, for a total of 10 electrons.
1 mole of methane = 6.023×1023 methane molecules
6.023×1023 methane molecules contain = 10×6.023×1023
(1 methane molecule = 10 e–)
= 6.023×1024 electrons
(b) The possible values of l and m1 are:
n = 3
Therefore, l = 0, 1, 2
For l = 0, m1 = 0
l = 1, m1 = -1, 0, +1
l = 2, m1 = -2, -1, 0, +1, +2
Q6:
(a) The 4f subshell of an atom contains 12 electrons. What is the maximum number of electrons having the same spin in it?
(b) Explain the meaning of 4p6.
(c) Write the electronic configuration of the atom with atomic number 20
Answer:
(a) The 4f subshell of an atom can maximum accommodate 14 electrons (e–) because they have 7 orbitals.
Given, 4f- subshell = 12 e–
Electronic configuration for 4f subshell:
| ↿⇂ | ↿⇂ | ↿⇂ | ↿⇂ | ↿⇂ | ↿ | ↿ |
|---|
7 electrons have the same spin in it and 5 electrons have the same spin. Hence, 7 is the maximum number of electrons having the same spin in it.
(b) If the p subshell has 6 electrons, it has a fully filled orbital. Hence its boiling point and ionisation enthalpy are both high, implying that removing an electron is difficult.
(c) The element with an atomic number of 20 is Calcium (Ca).
The electronic configuration of the atom using the Aufbau principle is
1s2, 2s2, 2p6, 3s2, 3p6, 4s2
It belongs to the 2nd group and 4th period.
Q7:
Kavita was playing a game with her friends. As a part of the game, they asked her to express a wish. She said that she wanted to be able to see the atom. Atomic dimensions are from 10-12 m and nucleus is 10-15 m; the visible range in the electromagnetic spectrum is for wavelengths in the range of 10-7m. As a student of chemistry,
- Describe how the world would look for Kavita if she is granted her wish.
- What value can you draw from this?
Answer:
- Kavita will be able to view atoms if her wish is granted. Her eyes would be modified to perceive particles that are considerably smaller than real-life objects, making it impossible for her to see well in the real world.
- The lesson learned from this experience is that we should think rationally before making wishes, and her desire to examine atoms demonstrates her passion for science.
Q8:
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.40.
Answer:
Tip: Molarity is a formula for calculating or measuring the concentration of a solution. It, like normalcy, relates the number of moles of solute per litre of solution. The ratio of the number of moles in the solute divided by the total number of moles present in the solution is known as the mole fraction of a solute.
Molarity (M) is one of the most used units for measuring the concentration of a solution. It represents the number of moles of solute per litre of solution (moles/Litre). The following is the molarity formula:
Molarity = Number of moles of solute
Volume of solution
Given,
Moles of ethanol = 0.40 mol
∴ Moles of water = 1 – 0.40 = 0.60 mol
Now, mass of ethanol = 0.40 mol x 46 g/mol = 18.4 g
Mass of water = 0.60 mol x 18 g/mol = 10.8 g
∴ Mass of the solution = 18.4 g + 10.8 g = 29.2 g
Assuming the density of water to be one.
Volume of the solution = Mass/Density
⇒ Volume of solution = 29.2/1 = 29.2 ml = 0.0292 L
Hence,
Molarity of the solution = No. of moles of solute/Volume of solution
⇒ Molarity = 0.40/0.0292 = 13.6M
Q9:
State Dalton’s law of partial pressure? A 5-lit vessel contains equal masses of methane and helium at 760 mmHg pressure. Find the partial pressure of each gas.
Answer:
The partial pressure of a gas in a mixture is the pressure exerted by that gas alone. We can utilise the ideal gas law to address problems with gases in a mixture if we have a mixture of ideal gases. According to Dalton’s partial pressures law, the overall pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases:
PTotal = Pgas 1 + Pgas 2 + Pgas 3…
The mole fraction of gas, 𝒙, can also be used to express Dalton’s law:
Pgas 1= 𝒙1PTotal
Now calculating the partial pressure:
Let m be the mass of two gases.
Number of moles of Helium (He) = Given mass/Molar mass = m/4
Number of moles of Methane (CH4) = m/16
∴ Total moles = m/4 + m/16 = 5m/16
𝒙He = (m/4)/(5m/16) = 4/5
𝒙CH4 = (m/16)/(5m/16) = 1/5
Now, total pressure = 760 mmHg
The partial pressure of gas 1 = Gas 1 mole fraction x Total pressure
∴ PHe = 4/5 x 760 = 152 x 4 = 608 mmHg
And, PCH4 = 1/5 x 760 = 152 mmHg
Thus, the partial pressure of each gas is 608 mmHg and 152 mmHg, respectively.
Q10:
The empirical formula of a compound is CH2O, and the vapour density of that compound is 45. Then find the molecular formula of that compound.
Answer:
The empirical formula is simply a condensed version of the molecular formula.
We know,
Molecular weight = 2 x Vapour density = 2 x 45 = 90.
Let the molecular formula be CxH2xOx.
∴ 90 = CxH2xO = 12x + 2x + 16x = 30x
So, x = 3
Thus, the molecular formula is C3H6O3.
Q11:
On complete combustion of 0.246 g of an organic compound gave 0.198 g of carbon dioxide and 0.1014g of water. Determine the percentage composition of carbon and hydrogen in the compound.
Answer:
Given,
Mass of the organic compound = 0.246 g
Mass of carbon dioxide (CO2) formed = 0.198 g
Mass of water (H2O) formed = 0.1014 g
(i) Percentage of Carbon:
1 mole of CO2 contains 1 g atom of carbon.
i.e., 44 g of CO2 contains carbon = 12 g
∴ 0.198 g of CO2 will contain carbon = 12/44 x 0.198 g = 0.054 g
This is the amount of carbon in 0.246 g of the compound.
∴ Percentage of carbon in the compound = 12/44 x 0.198 x 100/0.246 = 21.95%
(ii) Percentage of Hydrogen:
1 mole of H2O contains 2 g atoms of hydrogen.
i.e., 15 g of H2O containing hydrogen = 2g
∴ 0.1014g of H2O will contain hydrogen = 2/18 x 0.1014 g
This is the mass of hydrogen in 0.246 g of the compound.
∴ Percentage of hydrogen in the compound = 2/18 x 0.1014 x 100/0.246 = 4.58%
Thus, the percentage composition of carbon and hydrogen in the compound is 21.95% and 4.58%, respectively.
Q12:
Explain the main features of VSEPR Theory.
Answer:
To minimise electron-electron repulsion, pairs of electrons surrounding the core atom of a molecule or ion are positioned as far away as feasible under the Valence Shell Electron Pair Repulsion (VSEPR) theory. Sidgwick and Powell first proposed the hypothesis in 1940.
The VSEPR theory is based on the idea that the molecule will take on a shape that minimises electronic repulsion in that atom’s valence shell.
The following are the main postulates of VSEPR theory:
- One of the constituent atoms in polyatomic molecules (molecules made up of three or more atoms) is designated as the central atom to which all other atoms in the molecule are attached.
- The shape of the molecule is determined by the total number of valence shell electron pairs.
- The electron pairs tend to align themselves in such a way that the electron-electron repulsion between them is minimised while the distance between them is maximised.
- The electron pairs are concentrated on the surface of the valence shell in such a way that the distance between them is maximised.
- If the core atom of the molecule is surrounded by bond pairs of electrons, the molecule will be asymmetrically structured.
- The molecule will have a deformed shape if the centre atom is surrounded by both lone pairs and bond pairs of electrons.
- The VSEPR theory can be applied to any molecule’s resonance structure.
- Two lone pairs have the most repulsion, while two bond pairs have the smallest.
- When electron pairs close in on one other around the centre atom, they repel each other. The energy of the molecules increases as a result of this.
- When electron pairs are separated by a large distance, the repulsions between them are reduced, and the molecule’s energy is reduced.
Q13:
What is Hund’s rule of maximum multiplicity? Explain by taking an example of nitrogen.
Answer:
According to Hund’s rule, electron pairing will not occur in orbitals of the same subshell until each orbital is singly filled. Furthermore, electrons with parallel spin should be present in all of these singly occupied orbitals.
If we consider nitrogen, which has the atomic number of 7. 1s2 2s2 2p3 is the electronic configuration. Half of the p orbitals are filled. Three electrons and three p orbitals are present. This is due to the fact that the three electrons in the 2p subshell will first fill all of the vacant orbitals before combining with electrons in them.
Q14:
These people are not concerned with the health of other people. The first element in every group of representative elements shows properties different from the characteristic properties of the group.
- Name three such elements.
- Give two abnormal properties of each one of them.
Answer:
- Three such elements are Boron, Carbon, and Nitrogen.
- Boron:
(i) Boron is more difficult to work with than the other elements in its family because it is so tiny.
(ii) Boron only makes covalent compounds, whereas the rest of the family produces both ionic and covalent molecules.
Carbon:
(i) The carbon atom’s small size makes it easier to establish many bonds, and catenation is also possible due to its small size.
(ii) Carbon is best known for its ability to catenate, a property that is used in organic chemistry to investigate structures made up of catenated carbon atoms. Carbon is also recognised for its catenation ability.
Nitrogen:
(i) It forms multiple bonds due to its small size and high electronegativity. It exists as a triple-bonded diatomic molecule. They are held by van der Waals forces that are weak.
(ii) Catenation occurs in group 15 elements as well, but to a lesser amount than carbon. Phosphorous has the greatest proclivity for catenation. Nitrogen has the least propensity due to the N-N weak bond. Nitrogen can be arranged into chains of up to three N-atoms.
Q15:
Professor of Delhi University found that some scraps emit high energy radiations, which harmed a large number of people. There are certain elements like Co-60 that emit radiations on their own and this phenomenon is called radioactivity. There are three kinds of rays.
- Name the ray which is used to treat cancer.
- Give the source of γ-rays used for treating cancer.
- Discuss the values not possessed by people disposing of radioactive waste materials
Answer:
- High-energy radiation is used in radiation therapy to shrink tumours and kill cancer cells. Radiation used to treat cancer includes X-rays, gamma rays, and charged particles.
- Living cells can be killed by gamma radiation, and malignant tumours can be damaged. The strength of Gamma radiation diminishes exponentially with penetration depth. They cause malignant cells to die or multiply more slowly by damaging their DNA.
- The nuclear fuel cycle isn’t the only source of radioactive waste. Radioactive materials are widely employed in health, agriculture, and research, among other fields.
Q16:
Give reasons:
- Ethyne molecule is linear.
- Covalent bonds are directional, while ionic bonds are non-directional.
- The water molecule has a bent structure.
Answer:
- Because the two carbon atoms making the single(sigma) bond in ethyne have an angle of 180 degrees between them, they create a straight line. The molecule is linear. Both of these carbon atoms are sp hybridized and line up in a straight line.
- Because an ionic bond is formed by the electrostatic force between two opposite charges, the bonding direction is irrelevant. Covalent attraction is directional because it is directed in a specified direction and at an angle to the bonding atoms.
- This is owing to the fact that each molecule has a variable amount of electrons and the VSEPR (Valence Shell Electron Repulsion) theory. Because there are two lone pairs of electrons attached to the oxygen in a water molecule, the lone pairs push the hydrogen atoms together, causing the molecule to bend.
Q17:
How would you classify the state of chemical equilibrium in a chemical reaction based on the extent to which the reactions proceed?
Answer:
When a system reaches equilibrium, there are no more variations in the reactant and product concentrations; the reactions continue but at the same pace. Equilibrium does not necessarily indicate equal concentrations. However, it is conceivable.
A reaction’s equilibrium concentration position is said to be “far to the right,” meaning that virtually all of the reactants are consumed at equilibrium. If scarcely any product is created from the reactants, the equilibrium position is said to be “far to the left.”
Q18:
BARC at Trombay in Mumbai has five nuclear reactors which produce electricity. Boron rods are used as control rods to absorb neutrons in nuclear reactors, used for the production of electricity. Boron steel containers are used to dispose of nuclear waste materials safely. Metal borides are used as a protective shield.
- Give a reason for the absorption of neutrons by boron.
- Give methods to dispose-of nuclear wastes safely.
- Give harmful effects of nuclear radiation
Answer:
- Boron’s atomic structure makes it an excellent neutron absorber. The 10B isotope, which has a high nuclear cross-section and may catch thermal neutrons produced by uranium fission, has a natural abundance of roughly 20% and has a high nuclear cross-section.
- The radioactivity of the wastes decays with time, therefore storing high-level waste for around 50 years until disposal is a major incentive. Low-level garbage disposal is simple and may be done practically any place in a safe manner. Excess fuel is generally contained for at least five years underwater and then in dry storage. According to most experts, the best solution for the eventual disposal of the most radioactive waste produced is deep geological disposal.
- Acute health impacts such as skin burns and acute radiation syndrome (“radiation sickness”) can occur when people are exposed to extremely high quantities of radiation, such as when they are near an atomic blast. It can potentially have long-term health consequences, including cancer and cardiovascular problems.

Comments