Acetanilide is a white organic solid compound used primarily in organic synthesis. N-phenylacetamide, acetanilide and acetanil are other names of this compound. It was used in the past to treat fever and headache and was known as Antifebrin by its brand name.
Table of Contents
- Aim
- Theory
- Materials Required
- Apparatus Setup
- Procedure
- Crystallization
- Observations
- Results and Discussion
- Precautions
- Frequently Asked Questions– FAQs
Aim:
To prepare the organic compound acetanilide from aniline, glacial acetic acid/acetic anhydride and zinc dust.
| Also Read: Preparation of Acetanilide Viva Questions |
Theory:
Acetanilide is prepared from aniline when it reacts with acetic anhydride/glacial acetic acid in the presence of zinc dust. A mixture of aniline, glacial acetic acid, acetic anhydride and zinc dust is refluxed under anhydrous condition and then poured the mixture into ice cold water to get acetic anhydride precipitate. The crude precipitate of acetic anhydride is recrystallized to get pure crystals of acetanilide.
The chemical reaction is given below.
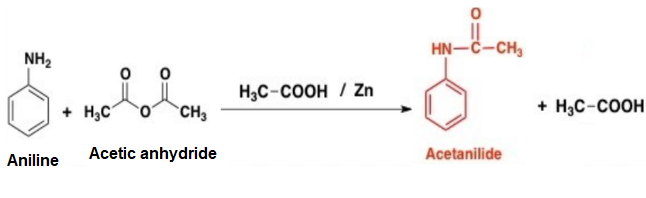
Zinc is used to prevent the oxidation of aniline during the chemical reaction. Acetanilide is medicinally important and it is used as febrifuge.
Acetanilide can also be prepared by acetylating aniline with acetic anhydride in the presence of concentrated hydrochloric acid. Dissolve aniline in hydrochloric acid and add acetic anhydride, then stir well. Pour the mixture of sodium acetate into water. Acetanilide is formed which can be separated and recrystallised by ethyl alcohol.
Other names – N-phenylacetamide, N-phenylethanamide, Acetanil
Materials Required:
- Aniline
- Glacial acetic acid
- Acetic anhydride
- Zinc dust
- Distilled water
- Round bottom flask
- Beaker
- Pipette
- Reflux condenser
- Funnel
- Stirrer
- Bunsen Burner
- Filter paper
- Electronic balance
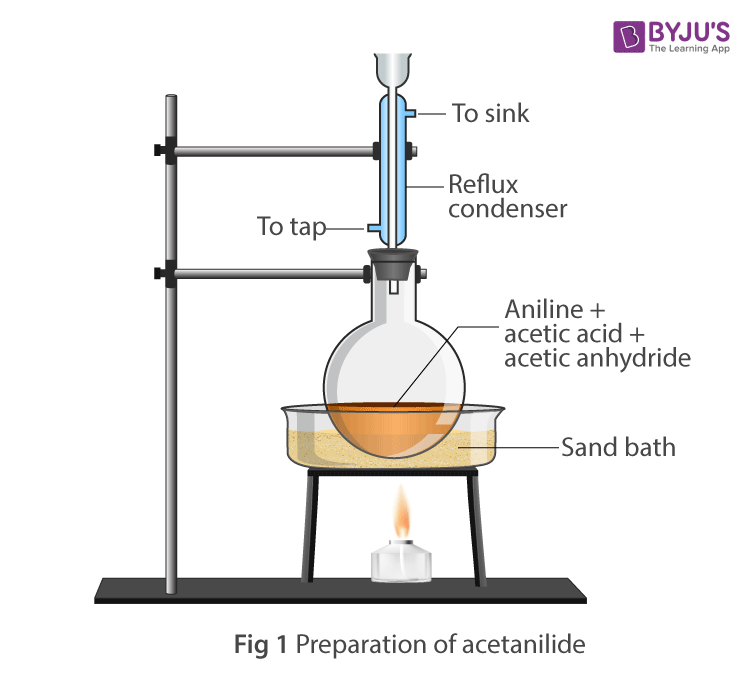
Apparatus Setup:
Procedure:
- Wash all the apparatus with distilled water before starting the experiment.
- Take a round bottom flask, and add 10ml of aniline, 20ml of acetic anhydride, glacial acetic acid mixture and zinc dust.
- Fix the reflux condenser with the round bottom flask.
- Heat the mixture gently for about 15-20 minutes in oil bath.
- Pour the hot mixture in a beaker containing ice cold water with constant stirring.
- Stir the mixture vigorously to hydrolyse excess of acetic anhydride.
- Once all the acetanilide is precipitated collect and filter in buchner funnel.
- The precipitate obtained is a crude sample of acetanilide. To get the pure crystals, crystallization should be carried out.
Crystallization:
Transfer the crude sample into a beaker containing 20ml water and heat gently. If the solution is coloured then add a small amount of activated carbon. Filter the hot solution with a funnel. Cool the mixture for 30 min so that white shiny crystals of acetanilide separate out. Filter off the crystals, wash them with water and dry in the folds of filter paper.
Observations:
| Colour of the crystals | Colourless crystals |
| Shape of the crystals | Plate shaped |
| Melting point | 114oC |
Results and Discussion:
The yield of Acetanilide is ______gm.
Precautions:
- Do not inhale the fumes of acetic anhydride.
- Always carry out experiments in fuming chamber or near the window.
- Use the water condenser for refluxing the reaction mixture.
- Dry the crystals of acetanilide before finding the weight and its melting point
Keep visiting BYJU’S to learn more about class 12 CBSE chemistry practicals.
Frequently Asked Questions on Preparation of Acetanilide
Name any two acetylating agent?
Acetic anhydride and acetyl chloride are the two acetylating agents.
What is the need to add zinc during the preparation of acetanilide?
Zinc is added to prevent the oxidation of aniline during the reaction. It reduces the coloured impurities present in the solution.
What is nitrating mixture?
The mixture of concentrated acids like nitric acid and sulphuric acid is called nitrating mixture.
What is the IUPAC name for acetanilide?
The IUPAC name for acetanilide is N-phenylacetamide
Mention any two uses of acetanilide.
Acetanilide is used in the synthesis of penicillin and in other pharmaceuticals. It is also used as an antipyretic agent means fever reducing agent.

How is zn metal form complex in this reaction?
In the formation of Acetanilide zinc prevent the oxidation of aniline during the reaction. It reduces the coloured impurities present in the solution. It does not form any complex compound.
What is the main role of glacial acetic acid?
Glacial acetic acid is nothing but anhydrous acetic acid. It acts as a source of acetic acid without the need for water as a solvent.