The elements present in group 13 of the modern periodic table are known as the Boron family (includes B, Al, Ga, In, Tl, Nh). These elements have 3 electrons in their outermost shell. Only one member of this family i.e. boron is typically a non-metal. The rest of the other elements are metals. Properties of these elements in the group vary greatly.
Physical Properties of Boron
The first element of the B family i.e., the boron itself has some physical properties different from that of the rest of the members. It is a hard and black-coloured non-metallic solid. The existence of boron can be seen in its many allotropic forms. It has an unusually high melting point because of the very strong crystal lattice. In contrast, the rest of the members are soft in nature and have low melting points. They have high electrical conductivity.
Chemical Properties of Boron
Oxidation states and trends in chemical reactivity:
Boron generally forms covalent bonds rather than +3 ions. This is due to the small size of boron which makes the sum of its first three ionization enthalpies very high. While moving from B to Al, the sum of the first three enthalpies drastically decreases. Due to a decrease in ionization energy from top to bottom down a group, the tendency to form Al3+ ion increases and also shows its high electropositive nature.
Moving down the group, the shielding effect decreases, this results in a higher effective nuclear charge. So the nucleus holds ns electrons tightly. Hence, only p orbital electrons are allowed for bonding. In Ga, In and Tl, oxidation states can be found as both +1 and +3. The relative stability of the +1 oxidation state can be seen as follows: Al< Ga< In < Tl reason being inert pair effect.
Compounds in the + 1 oxidation state are more ionic in comparison to the +3 oxidation state.
- Reactivity towards air: Aluminium forms a thin layer of oxide which makes it unreactive to other substances. Amorphous boron and aluminium when reacted with air by providing heat forms B2O3 and Al2O3 These elements are heated with dinitrogen to form nitrides. The chemical equation for the reaction of aluminium with air and dinitrogen is shown below:

The nature of the oxides varies as we move down the group. Boron trioxide is acidic in nature. Aluminium and gallium oxides are amphoteric in nature. Whereas indium and thallium are basic in nature.
- Reactivity towards acids and alkalis: Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Aluminium reacts with mineral acids and aqueous alkalis thus showing amphoteric character.
- The reaction of aluminium with HCl:
![]()
- The reaction of aluminium with aqueous alkali:
![]()
- Reactivity towards halogen: The boron family reacts with halogens to form trihalides (except TlI3)
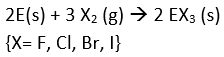 {X= F, Cl, Br, I}
{X= F, Cl, Br, I}
Frequently Asked Questions – FAQs
What are the properties of boron?
Either an amorphous dark brown to black powder or a dark, lustrous, and brittle crystalline metal occurs as a high purity boron. Extremely hard and resistant to heat, boron is a weak low-temperature conductor of electricity, but when temperatures increase, this improves.
What are the properties and uses of boron?
A dense amorphous powder is pure boron. As a jet fuel igniter, also in pyrotechnic bursts, amorphous boron is used. It brings a distinctive green colour to the flares. Boric (or boracic) acid, borax (sodium borate) and boric oxide are the most significant compounds of boron.
What is boron used for?
Boron is a mineral that’s present in the atmosphere and in food. Boron supplements are used by people as food. Boron is used to build solid bones, treat osteoarthritis, help build muscles and boost the level of testosterone, and to enhance cognitive skills and the synchronisation of muscles.
Why is boron so important?
Boron is an entity that is having multiple purposes. It is an essential plant nutrient, an important part of the nuclear industry, and the primary element of the bizarre fluid known as oobleck. Perched on the Periodic Table of Elements next to carbon, boron is a metalloid, a material with metallic as well as non-metallic properties.
What are some characteristics of the boron family?
By having three electrons in the outermost areas of their atomic structure, they are described as a group 13 elements. The lightest of these materials, Boron, is a metalloid. Silvery white metals include aluminium, gallium, indium, and thallium. Nihonium has only been formed in particle accelerators as individual atoms.
In this article, we briefly discussed the common properties of group 13 elements, Anomalous Properties of Boron. To know about the properties of other groups of elements in the periodic table, download BYJU’S – The Learning App.


Comments