Table of Contents
What is Red Phosphorus?
Red phosphorus is one of the most common allotropes of phosphorus and is considered to be a derivative of the P4 molecule. It exists in an amorphous (non-crystalline) network of phosphorus atoms. It is found to be more stable than white phosphorus (another naturally occurring phosphorus allotrope). Red phosphorus is characterized by its deep red colour and powdery texture.

White phosphorus undergoes a gradual transformation to yield the red phosphorus allotrope. This change occurs quicker in the presence of light and energy in the form of heat. When a sample of white phosphorus is partially converted into red phosphorus, it assumes a characteristic yellow appearance.
Structure
Red phosphorus exists in a polymeric chain of tetrahedrally structured P4 molecules in which one of the P-P bonds is broken to enable the linking of these tetrahedrons. The structure of red phosphorus is illustrated below.
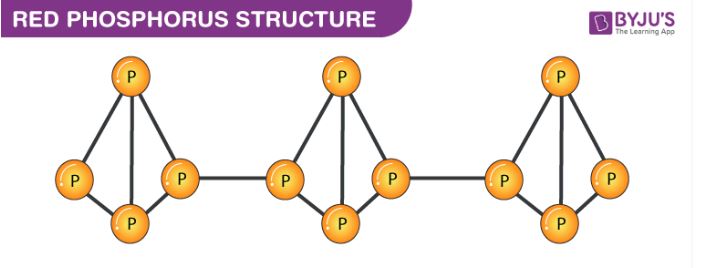
From the illustration provided above, it can be observed that the structure of red phosphorus is quite similar to that of a P4 molecule. Each phosphorus atom in P4 is linked to three other phosphorus atoms in a tetrahedral structure. When one of these bonds is broken, these tetrahedral structures can proceed to bond with neighbouring phosphorus atoms, resulting in a polymer-like structure.
Properties
Some important physical and chemical properties of red phosphorus are tabulated below.
| Molar Mass | 30.974 grams per mole |
| Red phosphorus formula | P4 (chain) |
| Density | 2.34 grams per cubic centimetre |
| Melting point | 860K |
| Phase (at standard temperature and pressure) | Solid |
Red phosphorus is odourless and has a deep red colour. It is not poisonous to humans, in contrast to the white phosphorus allotrope. Upon heating to temperatures above 300oC, red phosphorus undergoes crystallization. It can also assume a cubic structure in its crystal lattice.
This allotrope of phosphorus does not exhibit phosphorescence (a type of photo luminescence). Red phosphorus is not as chemically reactive as its white phosphorus counterpart.
Production
Red phosphorus was first discovered when Anton von Schrotter, an Austrian chemist, heated white phosphorus to 300oC. Some other important methods to obtain red phosphorus are discussed in this subsection.
From White Phosphorus
- The manufacture of red phosphorus involves the heating of white phosphorus (which must be submerged in water) to 550K in a steel pot for 3-4 days.
- The wastage of phosphorus in the form of vapour is prevented with the help of a system that condenses the reflux.
- After the 2-day mark, the temperature is increased to 673K in order to distil off the residual white phosphorus.
- After removing the water from the resulting mixture, the addition of sodium carbonate and the subsequent boiling of the mixture removes the remaining traces of the white allotrope.
- This mixture must now be vacuumed dried in order to obtain the red phosphorus product.
From Bone Ash or Phosphorus-Rich Rocks
- The first step of this method involves obtaining a finely ground powder from rocks that are rich in phosphorus or animal/fish bones.
- This powder/bone ash is treated with H2SO4 (sulphuric acid), yielding phosphoric acid along with some calcium sulfate.
- The heating of phosphoric acid with charcoal yields white phosphorus, which can be heated in order to obtain the required red phosphorus product.
The exposure of white phosphorus to sunlight results in a slow transformation that yields the red allotrope of phosphorus.
Applications
The striking surface of a matchbox is made up of red phosphorus and powdered glass. This mixture can be used to produce a spark that can light a matchstick. Some other important uses of red phosphorus are listed below.
-
- Many flares that are used as emergency signals use this allotrope of phosphorus to help in the ignition process. The sustained combustion of the flare is also achieved with the help of this allotrope.
- When mixed with magnesium and a binder, red phosphorus can be used as a smoke device that can quickly create a smoke screen.
- It is also used in the production of methamphetamine (commonly known as meth).
- Red phosphorus is also used as a flame retardant in many thermoplastics and thermosetting plastics.
Frequently Asked Questions – FAQs
What are the allotropes of phosphorus?
What is the structure of red phosphorus?
How is red phosphorus produced from white phosphorus?
What are the applications of red phosphorous?
To learn about important phosphorus compounds, such as phosphorous acid, register with BYJU’S and download the mobile application on your smartphone.

Comments