Introduction to Sulphur And Its Allotropic Forms
This topic focuses on Sulphur and its allotropic forms. In the periodic table sulphur is found in group 16. 0.17 % of earth’s crust consists of sulphur. It is a non-metal and is obtained as a by-product after the production of natural gas.
Table of Contents
The Allotropes of Sulphur
Sulphur forms numerous allotropes, but let us study the two most important allotropes of sulphur-
yellow rhombic sulphur (α-sulphur) and the monoclinic (β-sulphur). The most interesting feature is their thermal stability, the allotropes of sulphur are inter-convertible i.e. rhombic sulphur when heated above 369K gives monoclinic sulphur. Let us discuss these two allotropes in detail.
Rhombic sulphur (α-sulphur)
Rhombic sulphur is crystalline in nature and has an octahedral shape. On heating the solution of roll sulphur in CS2 we get rhombic sulphur. It is yellow with a melting point of 385.8K and specific gravity of 2.06. Rhombic sulphur cannot be dissolved in water but can be dissolved in benzene, ether, alcohol etc.
Monoclinic sulphur (β-sulphur)
When we take a dish and melt rhombic sulphur in that dish we obtain monoclinic sulphur after cooling it. In this process, we make two holes in the crust and pour out the remaining liquid. After this, we get colourless needle-shaped crystals of β-sulphur when the crust is removed.
Do you know why 369K is called transition temperature?
369K is called transition temperature because both the allotropes of sulphur are stable at this temperature. In other words, we can conclude that α sulphur is stable below 369K and it becomes β-sulphur above that temperature.
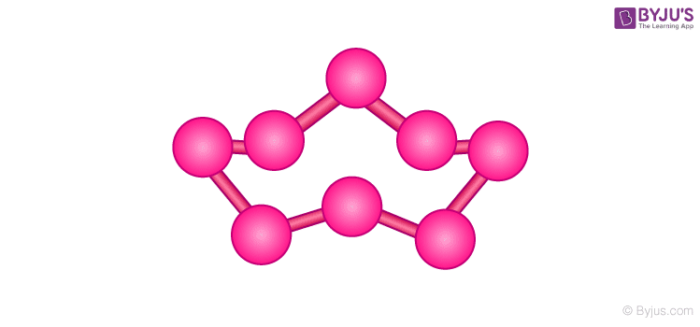
Rhombic and monoclinic sulphur, both have S8 molecules. The alternative packing of S8 molecules gives different crystal structures.
Uses of sulphur
-
-
-
- Sulphur is used for the vulcanization of rubber.
- Many of its compounds are used as insecticides in crops.
- Many bleaching agents can be manufactured using sulphur.
- It is also used in the manufacturing of carbon disulphide which in turn is used in skin ointments and other such products.
-
-
Sulphur has gained its importance because of its uses. Its uses are not just limited to the industries, it also plays a vital role in our ecosystem by affecting the growth of plants. This has led to the development of many sulphur containing fertilizers. Join BYJU’S to explore chemistry and its application.

Very good