Molecular geometry studies the three-dimensional shapes molecules form and how these shapes relate to chemical reactivity and physical characteristics. Atomic bonds and lone pairs of electrons give molecules their shapes. A molecule’s steric number is the sum of its bond pairs and lone pairs. The theory of valence shell electron pair repulsion (VSEPR) tries to explain the natural repelling forces of these electron arrangements.
According to electron geometry and VSEPR theory, tetrahedral shapes are formed by four “electron domains” (bonds or lone electron pairs around the central atom). The tetrahedral shape is formed by electrons repelling one another, resulting in the tetrahedron – the shape in which all electron pairs are as far apart as possible.
Table of Contents
- Tetrahedral Geometry Meaning
- Possible Shapes of Tetrahedron
- Examples of Tetrahedral Molecular Geometry
- Tetrahedral molecules with no central atom
- Frequently Asked Questions – FAQs
Tetrahedral Geometry Meaning
Tetra- denotes four, and -hedral refers to a solid’s face; “tetrahedral” literally means “having four faces.” When there are four bonds all on one central atom and no lone electron pairs, this shape is formed. The bond angles between the electron bonds are 109.5°, according to the VSEPR theory.
Methane is an example of a tetrahedral molecule (CH4). The four equivalent bonds in three dimensions point in four geometrically equivalent directions, corresponding to the four corners of a tetrahedron centred on the carbon atom.
Possible Shapes of Tetrahedron
Adding lone pairs to a molecule changes its shape in the geometries and the AXE methods. If the central atom contains one or more pairs of nonbonding electrons, the additional negative charge regions will behave similarly to the bonded atoms. The orbitals in the valence shell that contain the various bonding and nonbonding pairs will extend out from the central atom in directions that minimise their mutual repulsions.
0 Lone Pair
This molecule is composed of four equally spaced sp3 hybrid orbitals with bond angles of 109.5°. The orbitals have a tetrahedral shape. As there is an atom at the end of each orbital, the molecule has a tetrahedral shape.
The formula of the compounds with this molecular shape will be AX4.

1 Lone Pair
These molecules have trigonal pyramidal molecular geometries and are of the form AX3E.
Trigonal pyramidal is a molecular shape formed when three bonds and one lone pair exist on the central atom of the molecule. These molecules have sp3 hybridization at the central atom with tetrahedral electron pair geometries. The molecule ammonia (NH3) is trigonal pyramidal.

2 Lone Pairs
These are of the form AX2E2 and have bent shapes, which can be seen in the case of water.
This molecule is composed of four sp3 hybrid orbitals that form bond angles of approximately 104.5°. The orbitals have a tetrahedral molecular geometry. Two of the orbitals have lone pairs of electrons.
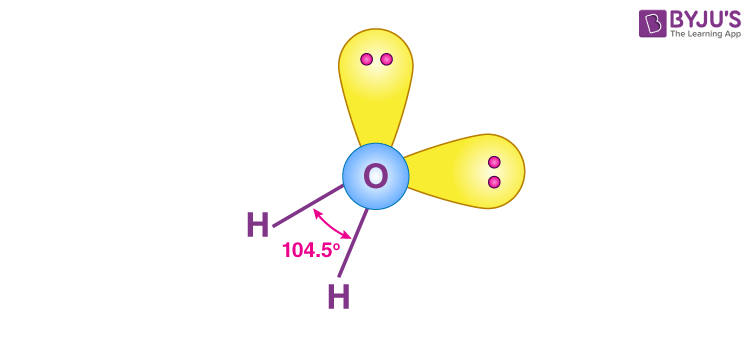
- Each of these molecules has four pairs of outer electrons surrounding the central atom. These electron pairs repel one another. In terms of repulsion’s relative strength:
| strongest | lone pair-lone pair |
|---|---|
| ↓ | lone pair-bond pair |
| weakest | bond pair-bond pair |
Examples of Tetrahedral Molecular Geometry
The tetrahedral molecular structure can be found in a variety of molecules, the most common of which is methane, CH4, Silane, SiH4, and thiazyl trifluoride, NSF3. The phosphate ion, (PO4)3-, the sulphate ion, (SO4)2-, and the perchlorate ion, (ClO4)– all have a tetrahedral structure.
Tetrahedral molecules with no central atom
There are a few molecules that have tetrahedral geometry but no central atom. Tetraphosphorus (P4) is an inorganic example, with four phosphorus atoms at the vertices of a tetrahedron, each bonded to the other three. Tetrahedrane (C4H4) is an organic example, with four carbon atoms each bonded to one hydrogen and the other three carbons. The theoretical C – C – C bond angle, in this case, is only 60°, indicating a high degree of strain.

Frequently Asked Questions on Tetrahedral Molecular Geometry
What factors contribute to tetrahedral shape?
Tetrahedral is a molecular shape that occurs when there are four bonds and no lone pairs in the molecule’s central atom. The atoms bonded to the central atom are located at the four corners of a tetrahedron, with 109.5° angles between them.
Are tetrahedral molecules planar?
A tetrahedral shape is not a planar shape, but it can be distorted by increasing the angle between two bonds.
What is the hybridization of tetrahedral compounds?
Tetrahedral compounds have sp3 hybridization.
Is tetrahedral symmetrical?
Tetrahedral molecules have no nonbonding electron pairs, and all bond angles are the same. As a result, the only way they can be asymmetric is if one atom differs from the others.
When a symmetrical nonpolar molecule is made asymmetric by replacing a surrounding atom with a new atom, the nonpolar molecule becomes polar if the new atom’s electronegativity differs significantly from that of the original atom.
What is the bond angle of a tetrahedral?
According to VSEPR Theory, the bond angle of a tetrahedral is 109.5°.


Comments