What is a Cyclic Process?
The process in which the initial and final states are the same is known as a cyclic process. It is a sequence of processes that leave the system in the same state in which it started.
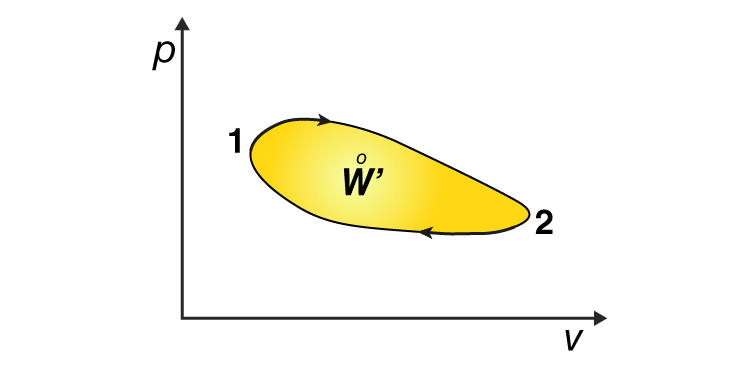
When a system undergoes a cyclic process, its initial and final internal energies are equal. Hence, the internal energy change in any cyclic process is zero. Applying the first law of thermodynamics to a cyclic process, we get,
Hence, the work done by the system in a cyclic transformation is equal to the heat absorbed by the system. The network involved in a cyclic process is the area enclosed in a P-V diagram. If the cycle goes clockwise, the system does work. If the cycle goes anticlockwise, then the work is done on the system every cycle. An example of such a system is a refrigerator or air conditioner. The Carnot engine is the best example of a cyclic process.
What is a Heat Engine?
A heat engine is a device by which a system is made to undergo a cyclic process that results in the conversion of heat to work.
- Heat engines consist of a working substance– the system. For example, steam in a steam engine is the working substance.
- The working substance goes through a cycle of several processes. In some processes, it absorbs heat from a reservoir at a high temperature.
- In some processes, the working substance releases some amount of heat to an external reservoir at a lower temperature.
- The work done by the system in a cycle is transferred to the environment through some arrangements. For example, the working substance may be in a cylinder with a moving piston that transfers mechanical energy to the wheels of the vehicle.
The schematic representation of a heat engine is represented as follows:

The cycle is repeated to get the useful work done for some purpose.
What is a Refrigerator?
A refrigerator is a reverse of the heat engine. Here, the working substance extracts some amount of heat from the cold reservoir at some temperature T1. External work W is done on it and heat Q is released to the hot reservoir at a temperature T2. The schematic representation of a refrigerator is given below.

Related Articles
| Carnot’s Theorem | Heat Engine Efficiency |
| Kelvin-Planck Statement | Clausius Statement |
Stay tuned to BYJU’S to learn more physics concepts like the working of refrigerator and heat pumps with the help of interactive videos.

Comments