Balz Schiemann Reaction Mechanism details the preparation and subsequent thermal decomposition of an aromatic fluoborate to give the corresponding aryl fluoride. The reaction gets its name from the German scientists – Günther Schiemann and Günther Balz.
Table of Contents
- Balz Schiemann Reaction
- Balz Schiemann Reaction Mechanism
Balz Schiemann Reaction
The basic reactants are aromatic amines, nitrous acid, and fluoroboric acid. Aromatic amines undergo diazotization under the influence of nitrous acid. Fluoroboric acid is now added to give rise to the corresponding aryl diazonium salt. Now, this aryl diazonium salt is subjected to heat to undergo thermal decomposition and give the aryl fluoride along with Nitrogen and Boron trifluoride.
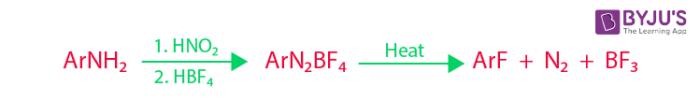
An example of such a process would be when a phenylamine is converted into a phenyl fluoride using fluoroboric acid, nitrous acid and the addition of heat as shown below:
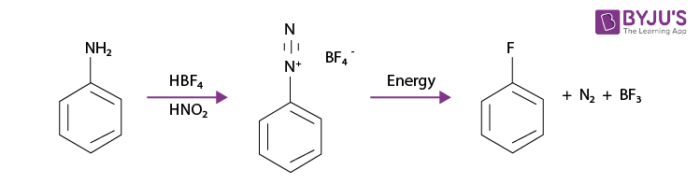
Balz Schiemann Reaction Mechanism
Step 1: Formation of Nitrosyl cation by the dehydration of nitrous acid.
Step 2: Aryl amine exposed to nitrosyl cation. After undergoing dehydration and its tautomerization, the aryl amine yields the aryl diazonium salt.
Step 3: Addition of fluoroboric acid to the aryl diazonium salt and the subsequent addition of energy finally gives aryl fluoride via a diazonium tetrafluoroborate intermediate stage.
The step-by-step process of Balz Schiemann Reaction mechanism is illustrated below:
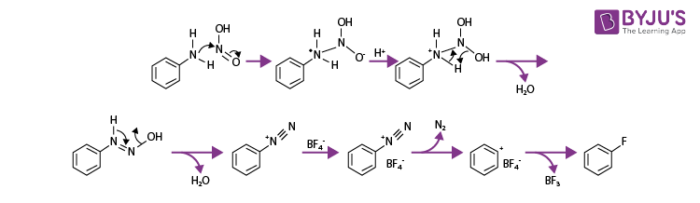
Counterions other than tetrafluoro borates can be used as well, for example – hexafluoro phosphates, hexafluoro antimonates. These counterions can also improve the yield for some substrates.
Thus, aryl fluorides can be easily prepared by the Balz-Schiemann reaction. It proves difficult to prepare aryl fluorides by the direct fluorination of aromatic hydrocarbons due to the violent nature of the reaction and the difficulty to control the reaction, making the Balz-Schiemann method the preferred way to get aryl fluorides. It is also the preferred way for some derivatives of fluorobenzene, 4-fluorobenzoic acid for example. However, the large scale thermal decomposition of these diazonium salts can lead to explosions.

Comments