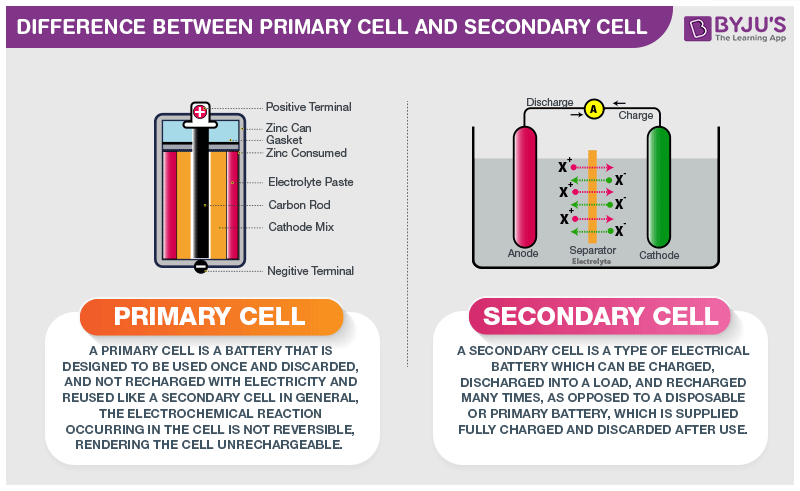
Primary Cell and Secondary Cell
Battery or cells are referred to as the parallel combination of electrochemical cells. The major difference between a primary cell and the secondary cell is that primary cells are the ones that cannot be charged but secondary cells are the ones that are rechargeable.
Primary cell
Primary cells have high density and get discharged slowly. Since there is no fluid inside these cells they are also known as dry cells. The internal resistance is high and the chemical reaction is irreversible. Its initial cost is cheap and also primary cells are easy to use.
Secondary cell
Secondary cells have low energy density and are made of molten salts and wet cells. The internal resistance is low and the chemical reaction is reversible. Its initial cost is high and is a little complicated to use when compared to the primary cell.
Difference Between Primary Cell and Secondary Cell
Primary cells are the ones which cannot be recharged and have to be discarded after the expiration of the lifetime whereas, secondary cells need to be recharged when the charge gets over. Both the types of battery are used extensively in various appliances and these cells differ in size and material used in them.
Difference Between Primary Cell and Secondary Cell |
|
|---|---|
|
Primary Cell |
Secondary Cell |
| Have high energy density and slow in discharge and easy to use |
They have smaller energy density |
|
There are no fluids in the cells hence it is also called as dry cells |
These are made up of wet cells (flooded and liquid cells) and molten salt (liquid cells with different composition) |
|
It has high internal resistance |
It has a low internal resistance |
|
It has an irreversible chemical reaction |
It has a reversible chemical reaction |
| Its design is smaller and lighter |
Its design is more complex and heavier |
| Its initial cost is cheap |
Its initial cost is high |
These were some difference between Primary cell and Secondary cell. If you wish to find out more, download BYJU’S – The Learning App.
Frequently Asked Questions – FAQs
Give some examples of secondary cells.
Lithium-ion battery and nickel-cadmium.
Give some examples of primary cells.
Alkaline batteries and dry cells are examples of primary cells.
What is the Function of a Salt Bridge in an Electrochemical Cell?
The salt bridge completes the circuit of an electrochemical cell, thereby allowing the flow of current through it. It also helps to maintain the overall electrical neutrality of the cell.
What is Standard Electrode Potential?
The standard electrode potential of an electrode can be defined as the potential difference that arises between the electrode and the electrolyte under standard conditions (Temperature = 298K, pressure = 1 atm, unity concentration of reacting species).
What are the Key Differences between Cathode and Anode?
The cathode of an electrochemical cell is the site at which reduction occurs. It is generally represented by a positive (+) sign. The electrons flow from the anode towards the cathode. In electrochemical cells, the anode is the electrode at which oxidation occurs. It is denoted by a negative (-) sign.
RELATED ARTICLES:
| Difference Between Cell and Battery | Fuel Cell |

nice a great job. keep it up.