An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions.
Table of Content
- What are Electrochemical Cells?
- Half Cells and Cell Potential
- Types of Electrochemical Cells
- Applications of Electrochemical Cells
What is an Electrochemical Cell?
An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it. These devices are capable of converting chemical energy into electrical energy, or vice versa. A common example of an electrochemical cell is a standard 1.5-volt cell which is used to power many electrical appliances such as TV remotes and clocks.
Such cells capable of generating an electric current from the chemical reactions occurring in them care called Galvanic cells or Voltaic cells. Alternatively, the cells which cause chemical reactions to occur in them when an electric current is passed through them are called electrolytic cells.
A diagram detailing the different parts of an electrochemical cell is provided below.
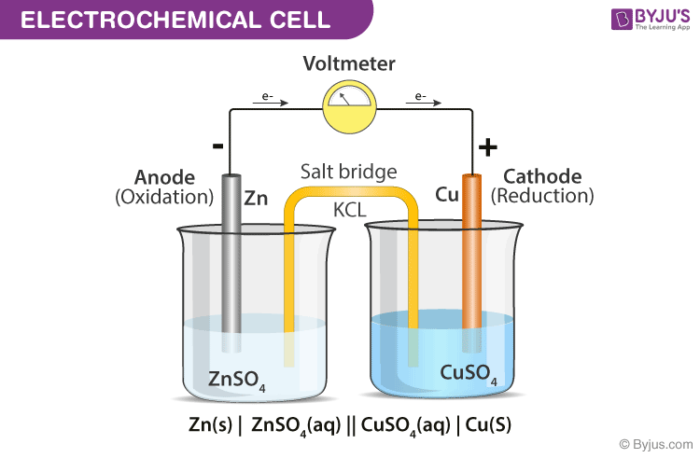
Electrochemical Cell
Electrochemical cells generally consist of a cathode and an anode. The key features of the cathode and the anode are tabulated below.
| Cathode | Anode |
| Denoted by a positive sign since electrons are consumed here | Denoted by a negative sign since electrons are liberated here |
| A reduction reaction occurs in the cathode of an electrochemical cell | An oxidation reaction occurs here |
| Electrons move into the cathode | Electrons move out of the anode |
General convention dictates that the cathode must be represented on the right-hand side whereas the anode is represented on the left-hand side while denoting an electrochemical cell.
Recommended Videos
Introduction To ElectroChemistry – Galvanic Cell

Electrochemistry – One Shot & Mind Maps

Conductors, Conductance & Conductivity
Galvanic Cell, Electrolytic Cell, EMF of Cell & Faraday’s Laws
ElectroChemistry Class 12 Chemistry – Important Topics for JEE Main

Half-Cells and Cell Potential
- Electrochemical Cells are made up of two half-cells, each consisting of an electrode which is dipped in an electrolyte. The same electrolyte can be used for both half cells.
- These half cells are connected by a salt bridge which provides the platform for ionic contact between them without allowing them to mix with each other. An example of a salt bridge is a filter paper which is dipped in a potassium nitrate or sodium chloride solution.
- One of the half cells of the electrochemical cell loses electrons due to oxidation and the other gains electrons in a reduction process. It can be noted that an equilibrium reaction occurs in both the half cells, and once the equilibrium is reached, the net voltage becomes 0 and the cell stops producing electricity.
- The tendency of an electrode which is in contact with an electrolyte to lose or gain electrons is described by its electrode potential. The values of these potentials can be used to predict the overall cell potential. Generally, the electrode potentials are measured with the help of the standard hydrogen electrode as a reference electrode (an electrode of known potential).
Primary and Secondary Cells
- Primary cells are basically use-and-throw galvanic cells. The electrochemical reactions that take place in these cells are irreversible in nature. Hence, the reactants are consumed for the generation of electrical energy and the cell stops producing an electric current once the reactants are completely depleted.
- Secondary cells (also known as rechargeable batteries) are electrochemical cells in which the cell has a reversible reaction, i.e. the cell can function as a Galvanic cell as well as an Electrolytic cell.
- Most of the primary batteries (multiple cells connected in series, parallel, or a combination of the two) are considered wasteful and environmentally harmful devices. This is because they require about 50 times the energy they contain in their manufacturing process. They also contain many toxic metals and are considered to be hazardous waste.
Types of Electrochemical Cells
The two primary types of electrochemical cells are
1. Galvanic cells (also known as Voltaic cells)
2. Electrolytic cells
The key differences between Galvanic cells and electrolytic cells are tabulated below.
| Galvanic Cell / Voltaic Cell | Electrolytic Cell |
| Chemical energy is transformed into electrical energy in these electrochemical cells. | Electrical energy is transformed into chemical energy in these cells. |
| The redox reactions that take place in these cells are spontaneous in nature. | An input of energy is required for the redox reactions to proceed in these cells, i.e. the reactions are non-spontaneous. |
| In these electrochemical cells, the anode is negatively charged and the cathode is positively charged. | These cells feature a positively charged anode and a negatively charged cathode. |
| The electrons originate from the species that undergoes oxidation. | Electrons originate from an external source (such as a battery). |
Applications of Electrochemical Cells
- Electrolytic cells are used in the electrorefining of many non-ferrous metals. They are also used in the electrowinning of these metals.
- The production of high-purity lead, zinc, aluminium, and copper involves the use of electrolytic cells.
- Metallic sodium can be extracted from molten sodium chloride by placing it in an electrolytic cell and passing an electric current through it.
- Many commercially important batteries (such as the lead-acid battery) are made up of Galvanic cells.
- Fuel cells are an important class of electrochemical cells that serve as a source of clean energy in several remote locations.
Frequently Asked Questions on Electrochemical Cells
What is the Function of a Salt Bridge in an Electrochemical Cell?
The salt bridge completes the circuit of an electrochemical cell, thereby allowing the flow of current through it. It also helps maintain the overall electrical neutrality of the cell.
What is Standard Electrode Potential?
The standard electrode potential of an electrode can be defined as the potential difference that arises between the electrode and the electrolyte under standard conditions (Temperature = 298K, pressure = 1 atm, unity concentration of reacting species). It is denoted by the symbol ‘Eocell‘. Click here to learn more about standard electrode potential.
What are the Key Differences between Cathode and Anode?
The cathode of an electrochemical cell is the site at which reduction occurs. It is generally represented by a positive (+) sign. The electrons flow from the anode towards the cathode. In electrochemical cells, the anode is the electrode at which oxidation occurs. It is denoted by a negative (-) sign.
Is it Possible for an Electrochemical Cell to have a Positively Charged Anode or a Negatively Charged Cathode?
Yes, the anode of an electrolytic cell is positively charged (and the cathode is negatively charged). However, oxidation still occurs at the anode despite the negative charge. The chemical reactions that occur in these electrochemical cells are non-spontaneous in nature.
What are electrolytic cells?
Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The chemical reaction that occurs inside such cells is commonly referred to as electrolysis. Electrolytic cells can be used to break down bauxite into aluminium and other components. Such cells can also be employed for the electrolysis of water into hydrogen and oxygen.
Thus, the key concepts and the types of electrochemical cells are discussed. To learn more about important concepts related to electrochemistry, such as the Nernst equation, register with BYJU’S and download the mobile application on your smartphone.

I love these website because i have gain allot from the website. I love the good doings keep it up.
I really like this website it is really good i learnt alot
I really like this website i have really gained alot.
i really love this site,it is very educative
i think its amazing i found it hard to learn electromagnetism
Thank you so much on this website. It is so helpful and information rich site. More power.
Thank you so much. This website has the best work for my learning
Here ,in the types of electrochemical cell ,how electrolytic cell be the type of electrochemical cell?it is totally different from the electrochemical cell .
Electrolytic cell is a type of electrochemical cell in which electrical energy is converted into chemical energy.
I really like this website .This website has the best work for my learning.