Introduction
Hunsdiecker Reaction is a chemical reaction that involves the silver salts of carboxylic acid reacting with halogens to form an unstable intermediate which further undergoes decarboxylation thermally leading to the formation of a final product known as alkyl halides.
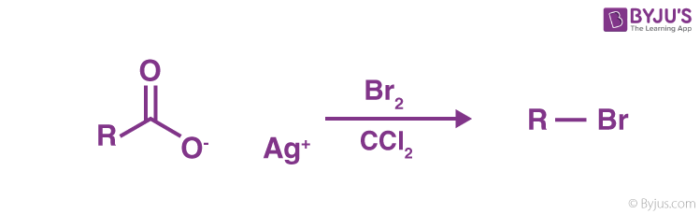
This reaction is also called Hunsdiecker–Borodin reaction or Borodin reaction. It is an example of both halogenation and decarboxylation reaction.
Table of Contents
- A Brief History
- Mechanism of the Reaction
- Some Variations of the Reaction
- Frequently Asked Questions – FAQs
A Brief History
Alexander Borodin was the first to demonstrate this type of reaction in the year 1861 with the preparation of methyl bromide using silver acetate. The mechanism was also later applied by Angelo Simonini, a pupil of Austrian- Jewish chemist Adolf Lieben while experimenting with the degradation of fatty acids which included reactions between iodine and silver carboxylates.
However, the name of the reaction has been specially kept after a German chemist, Heinz Hunsdiecker and his wife Clare Hunsdiecker. They basically improved the reaction and their contributions led to it being the general method to produce organic halide.
Mechanism of the Reaction
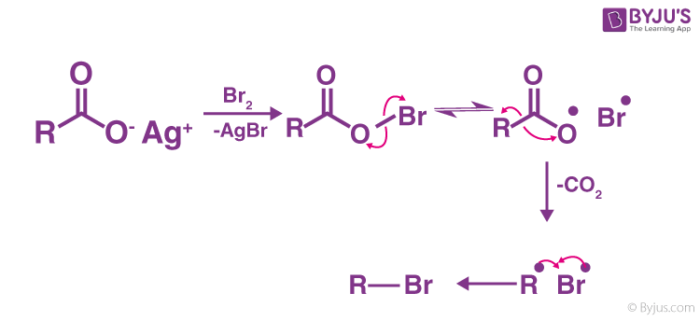
Hunsdiecker Reaction mechanism mainly involves organic radical intermediates where;
- Formation of the reactive intermediate.
- The occurrence of decarboxylation to form a diradical pair.
- Recombination of reactant to form the desired product.
To break down the process further, the reaction starts by heating silver carboxylate in CCl4 along with bromine. During this, the silver carboxylate is transformed into acyl hypobromite which is mainly due to the presence of bromine. Then precipitation of the stable silver bromide takes place. Consequently, a radical chain reaction takes place involving homolysis of the weaker oxygen-bromine bond. This results in the formation of a bromine atom and the carboxyl radical. This carboxyl radical decarboxylates, resulting in the formation of diradical pair of a hydrocarbon radical or an alkyl radical which then recombines to form the desired halide which in this case is an alkyl bromide.
Some Variations of the Reaction
Hunsdiecker’s reaction also has several variations. There are cases where silver(I) carboxylate is exchanged with thallium(I) carboxylate and the carboxylic acid is reacted or treated with halide ions of bromine, chloride or iodide and lead tetraacetate mainly to effect halogenation and decarboxylation. Similarly, in the preparation of 1-Bromo-3-chlorocyclobutane from 3-chlorocyclobutane carboxylic acid mercury oxide and bromide are used.
Frequently Asked Questions – FAQs
Which product is formed in the Hunsdiecker reaction?
Hunsdiecker Reaction is a chemical reaction involving the silver salts of carboxylic acid that react with halogens to create an unstable intermediate that further undergoes thermal decarboxylation leading to the formation of an alkyl halide-like final product.
Why CCl4 is used in the Hunsdiecker reaction?
The Hunsdiecker reaction (also referred to as the Borodin reaction or the Hunsdiecker-Borodin reaction) is an organic chemistry name reaction whereby carboxylic acid silver salts react to create an organic halide with a halogen. CCl4 only serves as a solvent that is actually used for the reaction ‘s smooth behaviour.
What does pyridine do in organic chemistry?
For carbonyl groups, pyridine is a reasonable nucleophile and is often used in acylation reactions as a catalyst. In pyridine, the nitrogen atom is nucleophilic so the lone pair of nitrogen electrons will not be delocalised around the ring.
What is SNi reaction mechanism?
A particular but not always observed nucleophilic aliphatic substitution reaction mechanism stands for SNi or Substitution Nucleophilic internal. Nucleophilic reactions occurring with retention of configuration were labelled in 1937, but were later used to identify different reactions that proceed with identical mechanisms.
Which amines are more basic?
Tertiary amines are more basic than secondary amines, which are more basic than primary amines, and finally ammonia is the least basic in gaseous state. The order of pKb’s (basicities in water) does not follow this order. In aqueous state secondary amines are more basic than primary amines and primary amines are more basic than tertiary amines. Similarly aniline is more basic than ammonia in the gas phase, but ten thousand times less so in an aqueous solution.

Comments