What is Hydrogen Fuel?
Hydrogen fuel is a zero-emission fuel when it is burned with oxygen.
Students interested in Chemistry should have certainly acquainted themselves with the term Hydrogen. Here we are going to discuss hydrogen fuel. Most of us are aware of the fact that the unique element of hydrogen has several similarities with both halogens and alkali metals. The popularity of hydrogen fuel is increasing day by day due to the reason that a large amount of heat is produced by this fuel on combustion.
Hydrogen Fuel Cell might be an astonishing fact for most of us to know that this fuel could release more energy in comparison to diesel or petrol. This means Hydrogen Fuel Cell has an efficiency three times more in comparison to petrol. Also, lesser pollutants are produced by the burning of this fuel than compared to the use of petrol.
Hydrogen is the main fuel, but fuel cells do require oxygen. One of the main appeals of fuel cells is that they generate electricity with very little pollution – much of the hydrogen and oxygen used to generate electricity ultimately combine to form an innocuous by-product, namely water. Each fuel cell also has an electrolyte that carries electrically charged particles from one electrode to the other, and a catalyst that accelerates electrode reactions.
Table of Contents
What is Hydrogen Fuel Cell?
Hydrogen Fuel Cells shows how these fuel cells work, how they came to be, and how they are coming into wider use.
The fuel contains nitrogen oxides as pollutants since hydrogen uses nitrogen as the impurity (here we are talking about the molecule of hydrogen and not the atom). The pollution can be brought down if a little water is added to the container to reduce the temperature. That would deter the reaction between oxygen and nitrogen.
Compared to a petrol tank containing the fuel, a hydrogen gas cylinder in compressed form would weigh much higher. To cool down the hydrogen gas into a liquid state, the temperature has to be brought down to 20 K. Due to these constraints, it is quite difficult to use this fuel efficiently. Hence, experts around the world are conducting research to find ways on how to use this fuel in a simple way in the future.
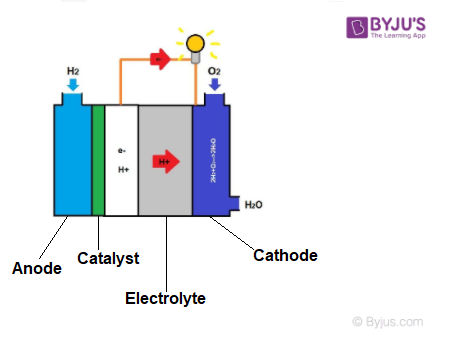
Hydrogen Fuel Cell – Explanation
Hydrogen and fuel cells (FCs) are usually considered inseparable because, among FCs, hydrogen-fueled FCs have the best performance and the lowest environmental impact. Hydrogen (like electricity) is not a primary source of energy, but rather a carrier of energy. There are no natural, pure hydrogen reservoirs. Water can be electrolyzed in the reverse of the fuel cell reaction to produce hydrogen. In order to force electrolysis to take place, considerable amounts of electricity are needed, much of which is currently generated by the burning of fossil fuels.
However, not all hydrogen fuel cells are intended for vehicles. Stationary fuel cells for the generation of electricity have been under development for decades. Some applications of fuel cells to residential or commercial buildings could involve the generation of electricity from fuel inputs such as natural gas or hydrogen and the use of waste heat from that process to heat the building.
Hydrogen atoms enter a fuel cell at an anode where they are stripped of their electrons by a chemical reaction. Hydrogen atoms are now “ionized” and have a positive electrical charge. Negatively charged electrons provide the current to work through wires. If alternating current (AC) is required, the DC output of the fuel cell must be routed through a conversion device called an inverter.
Recommended Videos
The source of hydrogen is commonly referred to as the fuel, and that gives its name to the fuel cell, although no combustion is involved. Then, hydrogen oxidation takes place in a very effective way, electrochemically. Hydrogen atoms react with oxygen atoms to form water during oxidation; electrons are released in the process and flow as an electric current through an external circuit.
A fuel cell is an electrochemical system combining hydrogen and oxygen to generate energy, with the by-products of water and heat. A single fuel cell, in its simplest form, consists of two electrodes-an anode and a cathode-with an electrolyte in between. Hydrogen interacts with a catalyst at the anode, producing a positively charged ion and an electron that is charged negatively.
Production of Hydrogen Fuel
Since pure hydrogen is not naturally available in large quantities on earth, it demands primary energy to obtain on an industrial level. Some frequent methods of production are steam methane reforming and electrolysis.
Production through Steam-methane reforming –
-
-
-
- Extraction of hydrogen from methane
- Liberating carbon dioxide and carbon monoxide into the atmosphere
- These gases help in the greenhouse effect and contribute to the change of climate.
-
-
Production through electrolysis –
-
-
-
- Separation of oxygen and hydrogen atoms
- This process can use solar, hydro, biomass, wind, geothermal, fossil fuels, and nuclear energy
- It is a cost-effective method.
-
-
Hydrogen Economy
-
-
-
- The term “hydrogen economy” or “economy of hydrogen” refers to the vision of using hydrogen as a low-carbon energy source. For example, replacing natural gas as a heating fuel or gasoline as a transport fuel.
- The main benefit of this scheme is that the energy transmission is done in the form of hydrogen instead of electric power.
- Presently, this fuel is used by mixing with CNG gas to improve its efficiency. It is expected to be used on a wider scale in the coming years. This fuel is also used in hydrogen fuel cells to create electricity.
-
-
Uses of Hydrogen Fuel
Hydrogen fuel can provide power for cars, aeroplanes, boats, and stationary or portable fuel cell applications, which can power an electric motor. It is very difficult to store hydrogen in either a cryogenic tank or a high-pressure tank which is the main problem of using hydrogen fuel cells in cars.
Thus we discussed hydrogen fuel and its importance and uses for the common man. Let us hope that this fuel would soon be used in our households in an economical manner over the coming years.
Advantages of Hydrogen Fuel:
-
-
-
- On burning hydrogen, it emits only water vapour,
- When hydrogen is burned it does not produce carbon dioxide.
- Hydrogen deletes little tailpipe pollution and is considered less of a pollutant.
- It has the ability to run a fuel-cell engine when compared to an internal combustion engine.
-
-
Frequently Asked Questions – FAQs
How efficient is a hydrogen fuel cell?
When the fuel cell is powered by pure hydrogen, it has the ability to be effective at up to 80%. That is, 80 per cent of the hydrogen energy content is converted into electrical energy. So we have an efficiency of 80% in producing electricity and an efficiency of 80% converting it to mechanical power.
How much power does a hydrogen fuel cell produce?
The power a fuel cell produces depends on several factors, including the type of fuel cell, the size, the temperature at which it operates and the pressure at which gas is supplied. A single fuel cell generates about 1 volt or less — barely enough electricity even for the smallest applications.
What are the advantages of a hydrogen fuel cell?
The hydrogen fuel cells are cleaner and more powerful than conventional engines and power plants based on combustion. Hydrogen and fuel cells can be used to drive cars and mobile power packs in mobile applications too. Fuel cells have the benefits of reducing greenhouse gas emissions.
What type of reaction is a hydrogen fuel cell?
A hydrogen fuel cell is an electrochemical cell that produces a current that can work using a spontaneous redox reaction. The net reaction to this is exothermic. The combination of the two half-cell potentials for the electrochemical reaction creates a positive potential for cells.
What is the disadvantage of hydrogen fuel cells?
As such, hydrogen plays an important role in the future as a substitute for imported petroleum, currently used in cars and trucks, but it has the following disadvantages. Although hydrogen cells are being used to power hybrid cars, it is still not a viable source of fuel for everyone. It’s costly./p>


Comments