What is Nitrogen trioxide?
N2O3 is a chemical compound formed by nitrogen and oxygen with the chemical name nitrogen trioxide. It is also called dinitrogen trioxide, or nitrogen sesquioxide. It is a highly toxic compound and an irritant mucous membrane.
Dinitrogen trioxide is a liquid which is blue and has an unpleasant, sharp odour. It has a long N–N bond having a bond length of 186 pm. It is a planar molecule which exhibits Cs symmetry. It dissociates partially into NO2 and NO. It extremely irritates to the skin, mucous membranes, and eyes. Inhaling the vapours can be very toxic.
Table of Contents
Properties of Nitrogen trioxide – N2O3
| N2O3 | Nitrogen trioxide |
| Molecular weight of N2O3 | 76.01g/mol |
| Density of Nitrogen trioxide | 1.447 g/cm3 |
| Boiling point of Nitrogen trioxide | 3.5 °C |
| Melting point of Nitrogen trioxide | −100.7 °C |
Nitrogen trioxide structure – N2O3
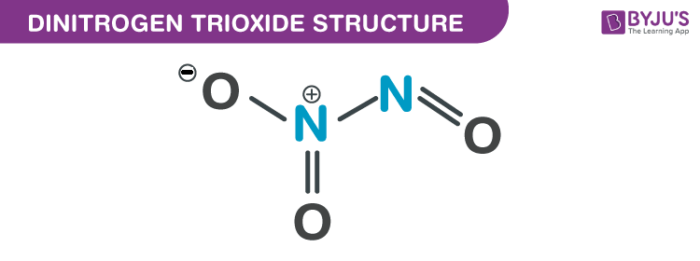
There are various other nitrogen oxides which possess long N–N bonds, that include dinitrogen tetroxide at 175 pm. The above image displays the dimensions taken from low-temperature, microwave spectroscopy.
It is produced as an anhydride when the unstable nitrous acid is mixed in water. If the nitrous acid (HNO2) can decompose into nitric acid and nitric oxide. Nitrite salts sometimes are obtained by adding N2O3 to the solutions of bases:
N2O3 + 2 NaOH → 2 NaNO2 + H2O
The mono isotopic mass and the exact mass of Nitrogen sesquioxide is 75.991 g/mol. The number of hydrogen bond donors is equals to zero whereas the number of hydrogen bond acceptors is equal to four. This compound is canonicalized and the number of covalently bonded units is equals to one.
Health hazards:
Nitrogen sesquioxide is a toxic and corrosive compound. It can be fatal if absorbed through the skin or inhaled. On heating the compound, it produces irritating, corrosive, toxic gases. When its liquid form comes in contact, it can cause severe injury and burns.
Learn more about the Structure, physical and chemical properties of N2O3 from the experts at BYJU’S.

Comments