What is Starch?
Starch is a tasteless, fluffy white powder that is insoluble in cold water, alcohol, and other solvents. Starch is a polysaccharide made up of 1,4 linkages between glucose monomers. The chemical formula of the starch molecule is (C6H10O5)n.
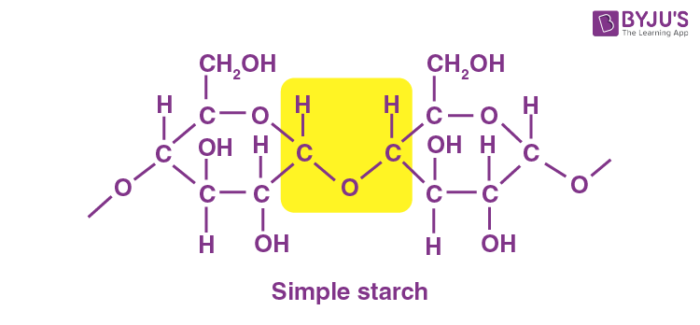
Starch is made up of long chains of sugar molecules that are connected together. The linear polymer amylose is the most basic form of starch, while amylopectin is the branched form. The primary role of starch is to help plants in storing energy. In an animal’s diet, starch is a source of sugar. Amylase, an enzyme contained in saliva and the pancreas that breaks down starch for energy, is used by animals to break down starch.
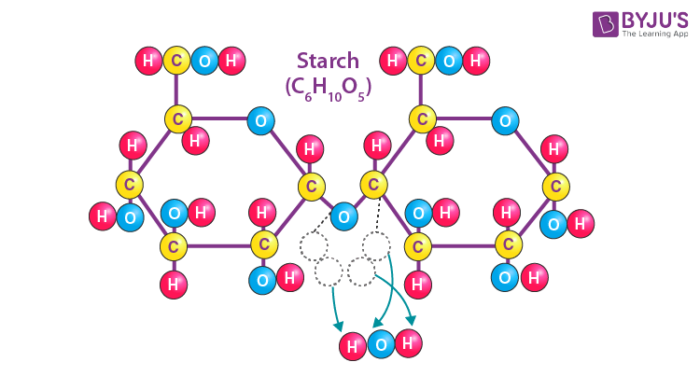
Table of Contents
- Preparation of Starch Solution
- General Properties of Starch
- Uses of Starch
- Recommended Videos
- Frequently Asked Questions
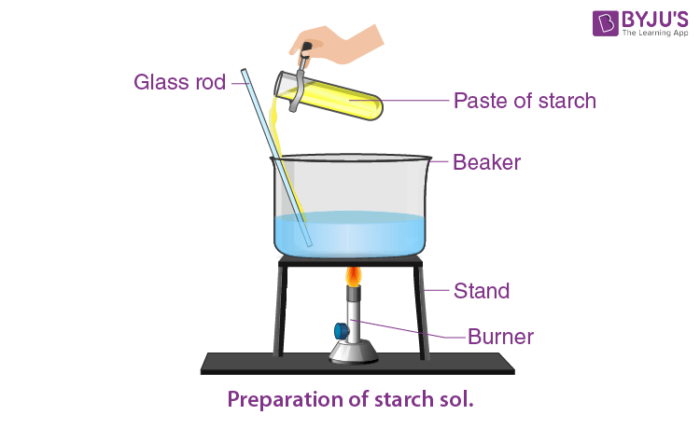
Preparation of Starch Solution
Commercial starch is made by following steps which are given below;
1. Crushing or grinding starch-containing tubers or seeds.
2. Combining the pulp with water.
3. Removal of any residual impurities.
4. Dry the resulting paste.
Steps for making a starch indicator solution:
1. Dissolve 1 gram of corn or potato starch in 10 mL distilled water, shake well, and pour into 100 mL boiling distilled water.
2. Boil the above solution formed for a minute and stir continuously.
3. Allow the solution some time for cooling.
4. In the last step, decant the supernatant and use it as the indicator solution if a precipitate forms.
The starch solution was prepared by heating in an autoclave at 125oC for 300 minutes., followed by rapid cooling in a water bath, and then homogenising in a warm blender for 30 seconds.
The starch solution thus prepared was examined under a microscope to confirm that all the starch granules had been ruptured to form a colloidal dispersion. In addition, a few preliminary flocculation tests were made with this solution and the results were compared with a rusticate starch solution. Virtually identical results justified the present preparation methods for the following tests unless stated otherwise. To minimise the effects of microbiological decomposition, fresh starch solutions were prepared each day. Chemically modified starches, both cationic and anionic, were solubilized in the same manner.
General Properties of Starch
The properties of starch are numerous. The structure of starch, especially amylose content, granule size distribution, granule form, granule-granule interaction, granule volume fraction, and continuous phase viscosity, all influence its rheological properties.
Starch has a number of properties.
- Starch as carbohydrate – Our main source of carbohydrates is starchy foods, which play an important role in a healthy diet. Potatoes, bread, rice, pasta, and cereals are examples of starchy foods.
- Starch as polysaccharide – Polysaccharides are a form of biological polymer that is widely used. In living organisms, their role is typically related to the structure or storage. In plants, starch (a polymer of glucose) is present in the forms of amylose and branched amylopectin and is used as a storage polysaccharide.
- Starch as a non-reducing sugar – It takes more than one hemiacetal “needle” in a haystack of “acetals” to give a positive sugar-reduction test. As a result, polysaccharides are not classified as reducing sugars. Starch, for example, results in a negative test. Starch and sucrose are both blue, indicating that they are non-reducing sugars.
Uses of Starch
- Foods that are high in starch are a good source of nutrition. They are broken down into glucose, which is the body’s main fuel, particularly for our brain and muscles, after they are ingested. B vitamins, iron, calcium, and folate are all essential nutrients found in starchy foods.
- Starch is widely used as a binder in the wet granulation process of massing and screening which is an important step in the production of tablets, capsules, and other solid dosage forms.
- The sole aim of starch in terms of dietary function is to turn glucose into energy for our body. Glucose is the only carbohydrate that our body can use. Glucose circulates in our bloodstream, where it is absorbed by cells and used as a source of energy.
- Food starches are commonly used as thickeners and stabilisers in foods like puddings, custards, soups, sauces, gravies, pie fillings, and salad dressings, as well as in the production of noodles and pasta.Starch is made up of long chains of sugar molecules that are connected together. Starch’s primary role is to help plants store energy. In an animal’s diet, starch is a source of sugar. Amylase, an enzyme contained in saliva and the pancreas that breaks down starch for energy, is used by animals to break down starch.
Recommended Videos

Frequently Asked Questions – FAQs
What is starch used for in chemistry?
Starch is used in a variety of industries, including the production of paper, textiles, pharmaceuticals, and biodegradable polymers, as well as a food additive.
Are Starch and Cellulose the same?
Starch and cellulose are two polymers that are very similar. They both are made of the same monomer, glucose, and have glucose-based repeating units. Only one difference is there between starch and cellulose. In starch, all the glucose repeat units are oriented in the same direction.
What is the difference between carbohydrate and starch?
The key difference between carbohydrates and starch is that carbohydrates can be polymeric or non-polymeric compounds, whereas starch is a polymeric carbohydrate.
Why is starch used as an indicator?
Since starch transforms to a deep dark blue colour when iodine is present in a solution, it can be used as a titration indicator. When starch is heated in water, it decomposes and develops beta-amylose. As beta-amylase reacts with iodine, it turns into a dark blue hue.
Why is the starch indicator added at the endpoint?
The Starch-Iodide complex is not very soluble in water, so the starch is added near the endpoint of an Iodine titration when the Iodine concentration is low. This eliminates errors due to the fact that some Iodine may remain adsorbed on the complex and go undetected.
Comments