Oxidation state of an element is defined as the degree of oxidation (loss of electron) of the element in achemical compound. Transition elements exhibit a wide variety of oxidation states in their compounds. For example: manganese shows all the oxidation states from +2 to +7 in its compounds. However, some elements exhibit few oxidation states, for example: Sc, Zn. The lower oxidation states exhibited by these elements is attributed to the fact that either they have few electrons to lose, for example Sc or too many d electrons (hence, fewer orbitals to share electron with others) for higher valence for example Zn. The variable oxidation states of transition elements arise mainly out of incomplete filling of d orbitals in such a way that their oxidation states differ from each other by unity.
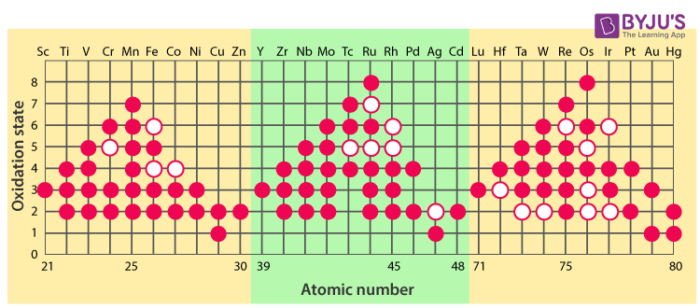
Stability of oxidation states
Higher oxidation states are shown by chromium, manganese and cobalt. In case of halides, manganese doesn’t exhibit +7 oxidation state, however MnO3F is known.Cu+2 (aq) is known to be more stable than Cu+ (aq) as the ΔhydH of Cu+2 is more than Cu+, which compensates for the second ionisation enthalpy of Cu. Mn exhibits high oxidation states in the oxides, for example:inMn2O7the oxidation state of Mn is +7. As oxygen is able to form multiple bonds with metal, Mn oxide, Mn2O7 shows a higher oxidation states in comparison to Mn fluorides, MnF4.In Mn2O7, each Mn is tetrahedrally surrounded by O’s including a Mn-O-Mn bridge. However, other elements of the group exhibit +3 oxidation states such as Fe2O3 and +4 oxidation state such as V2O4. In p-block elements we have seen lower oxidation states are favoured by the heavier members (due to inert pair effect) whereas, we acknowledge an opposite trend in d-block. As in group 6, Mo (VI) is found to have higher stability in comparison to Cr (VI).
For detailed discussions on oxidation states of transition elements, please visit BYJU’S.

Comments