AP Board 10th Standard Science Paper 1 Exam Question Papers 2015 with Solutions – Free Download
The AP SSC (Andhra Pradesh board) Class 10 Question Paper Science Paper 1 2015 with solutions is found in PDF format in this article, which the students can download and practise. This Class 10 exam question paper helps the students to prepare well for the Science exams and ace it. Students can easily get the PDF formats of the solved or even the unsolved question paper, by just clicking on the respective links. Students can refer to the solutions to check their progress in exam preparations.
AP SSC Science is a very interesting subject and students will have to be thorough with all the concepts of the subject, if they wish to crack it. Scoring high marks in the AP Board Class 10 Science exam is easy if a student has mastered the complex formulas and equations of Class 10 Science. To prepare more effectively for the exams, students are encouraged to solve the previous year papers of AP Board Class 10 Science. These AP SSC solved previous year papers give a complete overview of the question paper design, marking scheme and so on. This will help the students to know the several types of questions that can get repeated in the board exams. It will also help them to gauge the difficulty level of the final exams.
Download AP Board SSC 2015 Science Paper 1 Question Paper
Download AP SSC 2015 Science Paper 1 Question Paper With Solutions
AP Board Class 10 Science Paper 1 2015 Question Paper with Solutions
Part-A
SECTION – I 5×2=10
GROUP-A
1. State any two differences between Convex and Concave mirrors.
Answer: Concave mirrors, also known as converging mirrors are rounded or hollowed like the interior of a circle or a sphere, the Convex mirrors (diverging mirrors) are rounded or curved like the exterior of a sphere or circle. For Concave mirrors, the focus lies in front of the mirror i.e. focal length is positive, while for Convex mirrors, the focus lies behind the mirror and hence, it’s focal length is negative. The image formed by the concave mirror will be real, inverted and enlarged, except for when the object is between P and F. In this situation, the image is virtual, erect and enlarged. Sameways, the image formed by a Convex mirror is virtual, erect and diminished. Know more at Concave and Convex mirrors.
2. Write the Lens maker’s formula and explain the terms in it.
Answer: The lens maker’s equation for thin lenses is as given below;
1/ f = (n−1)(1/R1 – 1/R2), and here,
f is the focal length (half the radius of curvature)
n is the refractive index of the material used
R1 is the radius of curvature of sphere 1
R2 is the radius of curvature of sphere 2
Now, Learn about Lens Maker’s formula and derivation of lens maker’s formula from here.
3. What do you mean by electric shock? Explain how it takes place ?
Answer: The disturbance that occurs to the functioning of the organs inside a body, when a current of 0.0024A (or) P.D. 240v is passed through the body is known as Electric shock. The combined effect of potential difference caused by electric current and the resistance of the body is known as Electric shock. It occurs when electric current is passed through a person’s body. This happens, when a body comes in contact with any source for electrical energy and the circuit completes and current flows to the ground. Electric shock is experienced, when potential difference exists between one part of the body and another part. Resistance of the body will not be uniform throughout and it is likely that the current flowing through our body will select the path that has the least resistance. Hence, it can prove to be fatal if a large amount of current is passed through our body.
4. By observing steel vessels and different images in them, Ramu, a third class student asked some questions to his elder sister Santha. What may be those questions?
Answer: By observing some steel vessels and different images in them, Ramu, a third class student asked some questions to his elder sister Santha. They are as suggested:
- How do these steel vessels form images?
- Are these vessels mirrors?
- Why do the images differ from vessel to vessel?
- Why are the images formed on one side larger, while on the other side, the images formed are smaller?
- Why do the shape and size of the image depend on how we move away from the vessels?
- Why are the images not clear in the vessels?
GROUP-B
5. A mixture of Oxygen and Ethyne is burnt for welding. Can you tell why a mixture of Ethyne and air is not used?
Answer: When Ethyne is burnt in the air, it generates sooty flame as a result of incomplete burning or combustion, due to the limited supply of oxygen present in the air. Nevertheless, if ethyne is burnt at 3000o C with oxygen, then it gives off a clear flame because then the combustion will be complete. This oxy-acetylene flame is good for wielding, and it is not possible to get such a high temperature by burning ethylene in only air. For this reason, a mixture of ethylene and air is not used for welding.
6. Which method do you suggest for extraction of high reactivity metals? Why?
Answer: Highly reactive metals such as Sodium, Aluminium, Calcium, Potassium, Magnesium and so on are extracted from their ores most conveniently using the method of electrolysis. That is because, these highly reactive metals cannot be reduced by using Carbon or CO as these are less reactive. Electric current is passed through the ores to extract pure metals, in this method.
7. Write any two uses and two properties of Covalent compounds.
Answer: Covalent bonds formed by sharing an electron is highly directional in nature. These bonds are created as a result of effective orbital overlap between atoms. Covalent bonds are most likely to have lower melting and boiling points compared to ionic compounds. They also tend to be more flammable and their composition is hard and brittle. Covalent bonds also conduct electricity when dissolved in water.Two uses of covalent bonds include Diamonds(Jewellery) and Hydrocarbons(Fuels). Know more about Covalent Compounds and its properties.
8. Where do we use the washing method in our daily life ? Give an example. How do you correlate this example with enrichment of ore?
Answer: It is seen that we implement the washing method in our daily life, when we want to remove the impurities from our clothes, rice, cereals and so on. In this process, the ore particles are at first crushed and kept on a soapy surface, which are then washed using controlled flow of water. Impurities that are less dense, are washed away like this. Now, only the more dense ore particles remain. Meanwhile, enrichment of the ore is the removal of impurities or gangue from ore using various physical and chemical processes. The technique used for a particular ore depends on the difference in the properties of the ore and the gangue, thus correlating enrichment of ore to the example of using a washing method in daily life.
SECTION-II 4×1=4
9. What is Humidity ?
Answer: Humidity, an important aspect of atmosphere is the concentration of water vapour present in the air. This affects both climate and weather as well as global climate change. It also indicates the likely presence of precipitation, dew, fog and so on.
10. Define Critical angle.
Answer: Critical angle is the specific angle of incidence, beyond which total reflection of the light rays happen. The rays of light passing via a denser medium to a surface of lesser dense medium are not refracted any longer, but are reflected.
11. What is the reason for using Tungsten as a filament in an electric bulb?
Answer: For reasons for using tungsten as a filament in an electric bulb, you can check this answer here.
12. What is a Chemical bond ?
Answer: A lasting attraction between atoms, ions and compounds, which enables the formation of chemical compounds is known as Chemical bond. It also refers to the forces that hold the atoms together to form the molecules and solids.Thus, we can conclude that chemical bond is what keeps the atoms together to form the resulting compound.
13. Name the simplest Hydrocarbon.
Answer: Methane (CH4) is the simplest hydrocarbon.
14. Mention two methods, which produce very pure metals.
Answer: Methods used to produce pure metals include Electrolysis, smelting and reduction. These are the extraction processes employed in ore extraction to obtain pure metals.
SECTION-III 4×4=16
GROUP-A
15. Explain, why dogs pant during hot summer days usmg the concept of evaporation ?
Answer: Dogs pant during hot summer days so as to keep themselves cool despite the summer heat. If the surface area is larger, then the amount of evaporation is greater and also the area becomes more cool. Dogs have tongue with a large surface area. Meanwhile, the water molecules present on the surface of the tongue get evaporated, while panting, Thus, the area becomes cool by panting.
16.How can you verify that a current carrying wire produces a magnetic field with the help of an experiment ?
Answer: See, from here how the magnetic effect works. Check out the effect of a magnet on a current carrying wire.
17. Write about your favourite scientist/ physicist.
Answer: Sir Isaac Newton, an english mathematician, astronomer and physicist laid the foundation of classical mechanics. He also made seminal contributions to optics, and developed the infinitesimal calculus. Newton built the first practical reflecting telescope and developed a sophisticated theory of colour. He based his discovery on the observation that a prism decomposes white light into the colours of the visible spectrum. He also formulated an empirical law of cooling and made the first theoretical calculation of the speed of sound. Find more about Newton’s Laws of Motions from here.
18. A house has four tube lights, three fans and a television. Each tubelight draws
40 W. The fan draws 80 W and the television draws 100 W. On an average, all
the tubelights are kept on for 5 hours, all fans for 12 hours and the television
for 6 hours everyday. Find the cost of electric energy used in 30 days at the
rate of Rs. 3.00 per KWH.
Answer: Here, there are 4 tube lights, 3 fans and a TV
Power consumption of 1 tube light for an hour = 40 W
So, power consumption for 4 tube lights for an hour= 4 x 40 W= 160 W
Now, 4 tube lights are kept on for 5 hours.
Therefore, total power consumption by 4 tube lights = 5 x 160 W = 800 W
Then, take the fans. Power consumption of 1 fan per hour = 80 W
Hence, power consumption for 3 fans per hour= 3 x 80 W= 240 W
Here, 3 fans are kept on for 12 hours.
So, total power consumption for fans = 12 x 240 W = 2880 W
Finally, power consumption of TV per hour= 100 W
TV is kept on for 5 hours. So total power consumption = 5 x 100 W = 500 W
So, in a day the total power consumption will be = Lights + Fans + TV
= 800 W + 2880 W + 500 W
= 4180W = 4.180 KW
Therefore, total power consumption in 30 days at Rs. 3 per KW can be calculated as
= 4.180 x 30 x 3 = Rs. 376.20
GROUP-B
19. Write the balanced chemical reaction for the following and identify the type of reaction in each case.
(A) Magnesium(s) + lodine(g)➔ Magnesium iodide(s)
(B) Zinc(s)+ Hydrochloric acid(aq) ➔ Zinc chloride(aq) + Hydrogen(g),
Answer: (A) Magnesium(s) + lodine(g)➔ Magnesium iodide(s)
Add 2 here, to balance the equation, you get
2 Mg + I2➔ MgI2
This is an oxidation reaction: Magnesium loses 2 electrons to form a Mg2+ ion, while Iodine gains 1 electron and forms an I- ion. Each magnesium atom reacts with 2 iodine atoms. The oppositely ions attract and form a giant ionic lattice.
Magnesium Iodide, MgI2 is an ionic compound. It is prepared by mixing Mg metal with Iodine under strict anhydrous conditions. This is highly soluble in water, while it is highly unstable at normal temperatures in air. It is stable only when kept in an atmosphere of hydrogen gas.
(B) Zinc(s)+ Hydrochloric acid(aq) ➔ Zinc chloride(aq) + Hydrogen(g),
Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g)
Single replacement reaction: zinc metal displaces the hydrogen to form hydrogen gas and zinc chloride, a salt. Zinc reacts quickly with the acid to form bubbles of hydrogen.
20. Rainbow is an example for continuous spectrum. Explain
Answer: A natural spectrum, appearing in the sky right after the rain shower is known as a Rainbow. This is caused by the dispersion of the sunlight by tiny droplets of water that is present in the atmosphere. Meanwhile, a continuous spectrum contains various colours of different wavelengths with no gap between them. Also, in a rainbow, there are no sharp boundaries between the colours and so hence, they are known as examples for continuous spectrum. Learn more about how the rainbow is formed from here.
21. What is meant by water of crystallisation of a substance? Describe an activity to show the water of crystallisation.
Answer: Water crystallisation is the fixed number of molecules present in one formula unit of salt. Meanwhile, for the activity that shows water of crystallisation, substances required are Hydrated copper sulphate, bunsen burner, test tube.
Take some copper sulphate crystals in a test tube and heat it using the bunsen burner. You will observe that the blue colour crystals of the Copper sulphate have changed colour to white powder on removal of 5 molecules of water of crystallisation.
CuSO4.5H20 → CuSO4
22. Comment on the position of Hydrogen in the Periodic table.
Answer: Hydrogen is the first element of the periodic table because its atomic number is one, which means it has only one electron in its atom and so only one electron is present in its outermost shell. Know more about the position of hydrogen in the periodic table from here.
SECTION-IV 1×5=5
23. Draw a neat diagram of an A.C. generator.
Answer:
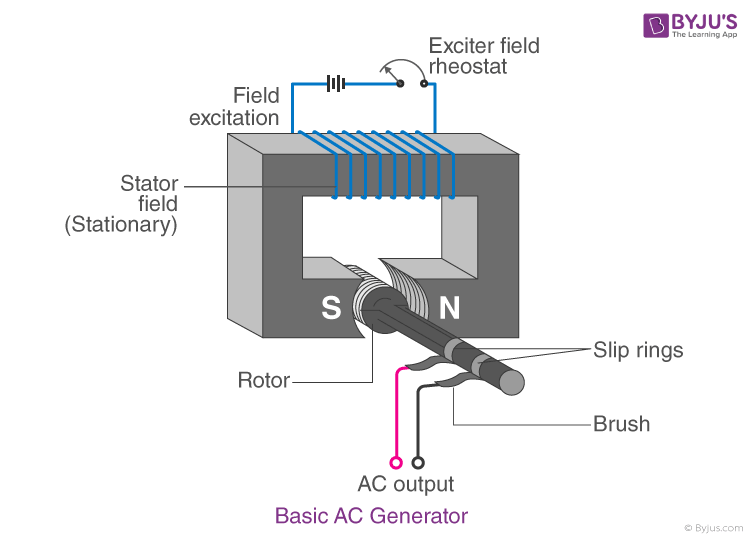
24. Draw a neat diagram of a Reverberatory furnace and label its parts.
Answer:
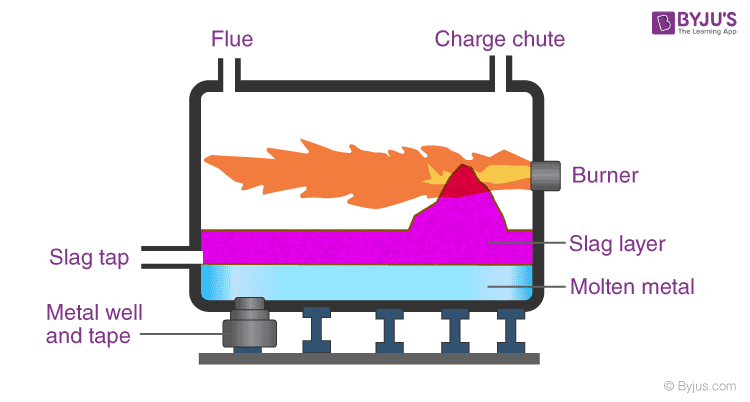




Comments