Before understanding periodic trends in electronegativity in the modern periodic table, let us first understand the term electronegativity. When an element combines with other elements to form bonds, sharing of electrons takes place. This sharing of electrons can be understood with the example of sharing of chocolates between children. Sometimes children share their chocolates equally, sometimes unequally. Some children share their chocolates easily but some do not. In the same way, some atoms share their electrons easily and some do not share their electrons easily. This sharing of electrons to form a bond depends on the electronegativity difference.
Electronegativity is defined as the ability of an element to attract the bonding pair of electrons towards itself. Although some numerical scales have been defined to measure electronegativity of elements in the periodic table like Pauling scale, Allred- Rochow scale and Mulliken-Jaffe scale, measurement of electronegativity is very difficult. Pauling scale is widely used to measure electronegativity. Atoms having high electronegativity attract more electrons and form bonds. The atoms with low electronegativity can easily lose their electrons. According to Pauling scale fluorine has the highest electronegativity in the periodic table and its value is 4.0, whereas caesium and francium are the least electronegative elements with values on the Pauling scale as low as 0.7.
Trends in electronegativity across a period:
When we move from left to right in a period of the modern periodic table, electronegativity increases. We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine. In this graph, we have not shown argon as it does not react with elements to form bonds.
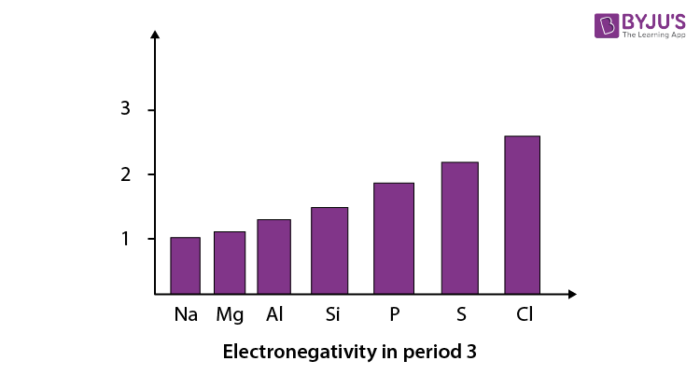
Trends in electronegativity in groups:
When we go down in a group in the periodic table, electronegativity decreases. The trend in electronegativity can be seen by the graph given below for group 7. Here fluorine has the highest electronegativity (4.0) . The trend is shown below.
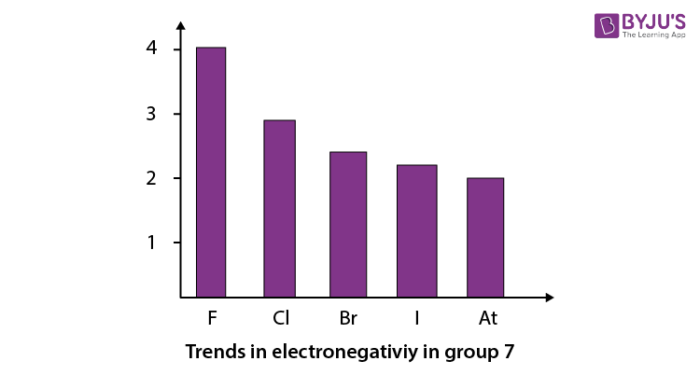
Recommended Videos
Electronegativity – Periodic Table

This was just a brief layout of the trends in electronegativity of an element in the periodic table. To know about the trends in other properties of elements across periods and groups of the periodic table subscribe to the BYJU’S YouTube channel.

Comments