Metals are natural compounds of earth’s crust, in which they are generally found in the form of metal ores, associated both with each other and with many other elements. They are also naturally present in the rocks washed by surface water and groundwater and in atmospheric dust.
Table of Content
- What are Metals?
- Examples of Metals
- List of all Metals
- Properties of Metals
- Frequently Asked Questions – FAQs
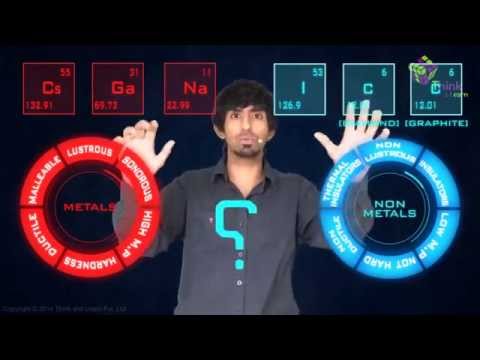
What are Metals?
Metals are substances that form naturally below the surface of the Earth. Most metals are lustrous or shiny. Metals are inorganic, which means they are made of substances that were never alive.
Examples of Metals

Metal is very strong and durable and therefore is used to make many things. These are used for making automobiles, satellites, cooking utensils, etc.
Most metals are hard but some are not. Sodium and potassium are such metals that can be cut by knife whereas mercury is a liquid metal at room temperature. Iron is solid in nature.
Recommended Videos
Types of Metals (The Complete List)
When all of the metals are to be considered as a group, an advantage may be gained if their names are set down in the tabular form.
| S.No | Atomic Number | Symbol | Metal Elements |
| 1 | 3 | Li | Lithium |
| 2 | 4 | Be | Beryllium |
| 3 | 11 | Na | Sodium |
| 4 | 12 | Mg | Magnesium |
| 5 | 13 | Al | Aluminium |
| 6 | 19 | K | Potassium |
| 7 | 20 | Ca | Calcium |
| 8 | 21 | Sc | Scandium |
| 9 | 22 | Ti | Titanium |
| 10 | 23 | V | Vanadium |
| 11 | 24 | Cr | Chromium |
| 12 | 25 | Mn | Manganese |
| 13 | 26 | Fe | Iron |
| 14 | 27 | Co | Cobalt |
| 15 | 28 | Ni | Nickel |
| 16 | 29 | Cu | Copper |
| 17 | 30 | Zn | Zinc |
| 18 | 31 | Ga | Gallium |
| 19 | 37 | Rb | Rubidium |
| 20 | 38 | Sr | Strontium |
| 21 | 39 | Y | Yttrium |
| 22 | 40 | Zr | Zirconium |
| 23 | 41 | Nb | Niobium |
| 24 | 42 | Mo | Molybdenum |
| 25 | 43 | Tc | Technetium |
| 26 | 44 | Ru | Ruthenium |
| 27 | 45 | Rh | Rhodium |
| 28 | 46 | Pd | Palladium |
| 29 | 47 | Ag | Silver |
| 30 | 48 | Cd | Cadmium |
| 31 | 49 | In | Indium |
| 32 | 50 | Sn | Tin |
| 33 | 55 | Cs | Cesium |
| 34 | 56 | Ba | Barium |
| 35 | 57 | La | Lanthanum |
| 36 | 58 | Ce | Cerium |
| 37 | 59 | Pr | Praseodymium |
| 38 | 60 | Nd | Neodymium |
| 39 | 61 | Pm | Promethium |
| 40 | 62 | Sm | Samarium |
| 41 | 63 | Eu | Europium |
| 42 | 64 | Gd | Gadolinium |
| 43 | 65 | Tb | Terbium |
| 44 | 66 | Dy | Dysprosium |
| 45 | 67 | Ho | Holmium |
| 46 | 68 | Er | Erbium |
| 47 | 69 | Tm | Thulium |
| 48 | 70 | Yb | Ytterbium |
| 49 | 71 | Lu | Lutetium |
| 50 | 72 | Hf | Hafnium |
| 51 | 73 | Ta | Tantalum |
| 52 | 74 | W | Tungsten |
| 53 | 75 | Re | Rhenium |
| 54 | 76 | Os | Osmium |
| 55 | 77 | Ir | Iridium |
| 56 | 78 | Pt | Platinum |
| 57 | 79 | Au | Gold |
| 58 | 80 | Hg | Mercury |
| 59 | 81 | Tl | Thallium |
| 60 | 82 | Pb | Lead |
| 61 | 83 | Bi | Bismuth |
| 62 | 84 | Po | Polonium |
| 63 | 87 | Fr | Francium |
| 64 | 88 | Ra | Radium |
| 65 | 89 | Ac | Actinium |
| 66 | 90 | Th | Thorium |
| 67 | 91 | Pa | Protactinium |
| 68 | 92 | U | Uranium |
| 69 | 93 | Np | Neptunium |
| 70 | 94 | Pu | Plutonium |
| 71 | 95 | Am | Americium |
| 72 | 96 | Cm | Curium |
| 73 | 97 | Bk | Berkelium |
| 74 | 98 | Cf | Californium |
| 75 | 99 | Es | Einsteinium |
| 76 | 100 | Fm | Fermium |
| 77 | 101 | Md | Mendelevium |
| 78 | 102 | No | Nobelium |
| 79 | 103 | Lr | Lawrencium |
| 80 | 104 | Rf | Rutherfordium |
| 81 | 105 | Db | Dubnium |
| 82 | 106 | Sg | Seaborgium |
| 83 | 107 | Bh | Bohrium |
| 84 | 108 | Hs | Hassium |
| 85 | 109 | Mt | Meitnerium |
| 86 | 110 | Ds | Darmstadtium |
| 87 | 111 | Rg | Roentgenium |
| 88 | 112 | Cn | Copernicium |
| 89 | 113 | Nh | Nihonium |
| 90 | 114 | Fl | Flerovium |
| 91 | 115 | Mc | Moscovium |
| 92 | 116 | Lv | Livermorium |
Physical Properties of Metals
- All the metals are good conductors of heat and electricity. Cooking utensils and irons are made up of metals as they are good conductors of heat.
- Ductility is the ability of the material to be stretched into a wire. This ability allows metals to be drawn into wires and coupled with their durability, find applications as cable wires and for soldering purposes. Because Metal can be drawn into wires we can say that metals are ductile.
- Malleability is the property of substances which allows them to be beaten into flat sheets. Aluminium sheets are used in the manufacturing of Aircrafts because of their lightweight and strength. Other metal sheets are used in automobile industries, for making utensils, etc. Therefore, metals are malleable.
- Metals are sonorous because they produces a deep or ringing sound when struck with another hard object.
- Usually, all the metals have a shiny appearance but these metals can also be polished to have a shiny appearance.
Chemical properties of Metals
- Reaction with water: Only highly reactive metals react with water and not all the metals. For example, Sodium reacts vigorously with water and oxygen and gives a large amount of heat in the process. This is why sodium is stored in kerosene so that it does not come in contact with moisture or oxygen.
- Reaction with acids: Hydrogen gas is produced when metals react with acids. For example, when zinc reacts with hydrochloric acid it produces zinc chloride and hydrogen gas.
- Reaction with bases: Not all the metals react with bases and when they do react, they produce metal salts and hydrogen gas. When zinc reacts with strong sodium hydroxide it gives sodium zincate and hydrogen gas.
- Reaction with oxygen: Metal oxides are produced when metals burn in the presence of oxygen. These metal oxides are basic in nature. For example: When a magnesium strip is burned in the presence of oxygen it forms magnesium oxide and when magnesium oxide dissolves in water it forms magnesium hydroxide.
Frequently Asked Questions – FAQs
What are metals?
A metal in chemistry is an element that creates positive ions (cations) readily and has metallic bonds. Metals are sometimes represented by a cloud of delocalized electrons and a lattice of positive ions.
What is metal or non-metal?
A metal is a component, material, or alloy that is usually solid, transparent, shiny, and has good conductivity in terms of electrical and thermal. Metals are usually malleable, which ensures that they can be indefinitely hammered or pressed without fracturing or cracking, as well as fusible or ductile
What is the hardest metal in the world?
Tungsten is the hardest metal with highest tensile strength, but it is brittle and appears to break down on contact.
What is the softest metal?
Caesium is considered to be the softest metal, and Lead is considered one of the softest metals. Mercury at room temperature is liquid (molten). Gallium is liquid at body temperature, while solid (if soft) at room temperature.
What happens if you touch calcium metal?
Calcium will react with water or moisture causing heat. If calcium comes in touch with any parts of body and eyes, it causes irritation and corrosion
For any queries contact our BYJU’S mentors.


OH MEANS HYDROXIDE?
Hydroxide is a diatomic anion with chemical formula OH⁻. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst.
WHAT IS THE ZINCATE SYMBOLIC FORMULA?
Zincate usually the anion Zn(OH)₄^(2−), more properly called tetrahydroxozincate or salts thereof, such as sodium zincate Na Zn(OH). The formula for sodium zincate is Na₂Zn(OH)₄.