Table of Contents
- What is Rearrangement Reaction?
- Recommended Videos
- Curtius Rearrangement or Curtius Reaction
- Claisen Rearrangement
- Beckmann Rearrangement
- Hofmann Rearrangement
- Pericyclic Rearrangement
- Photochemical rearrangements
- Frequently Asked Questions on Rearrangement Reaction
What is Rearrangement Reaction?
The term “rearrangement” is used to describe two different types of organic chemical reactions. A rearrangement may involve the one -step migration of an H atom or of a larger molecular fragment within a relatively short lived intermediate.
On the other hand, a rearrangement may be a multi-step reaction that includes the migration of an H atom or of a larger molecular fragment as one of its steps.
In many rearrangements, the migrating group connects to one of the direct neighbours of the atom to which it was originally attached. Rearrangements of this type are the so-called [1,2] – rearrangements or [1,2] – shifts. These rearrangements can be considered as sigma-tropic processes, the numbers 1 and 2 characterizing the subclass to which they belong.
Recommended Videos
Types Of Organic Reactions

Ring Expansion via Carbonation Rearrangement
Curtius Rearrangement or Curtius Reaction
Curtius’ reaction involves the heating of an acyl azide which loses nitrogen and then rearranges to an isocyanate.
RCON3 → R-N=C=O + N2
If the reaction is performed in an alcoholic or aqueous medium, the isocyanate further reacts to form urethane, amine or substituted urea.
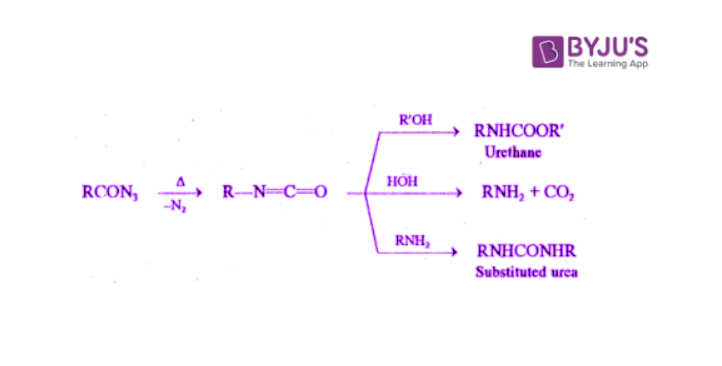
The conversion of acyl azides to isocyanates involves Curtius rearrangement whereas Curtius reaction involves the conversion of acids to amines, urethane and substituted urea via Curtius rearrangement.
Acyl azide required for the reaction is obtained as follows.
RCOCl + NaN3 (Sodium azide) → RCON3 (Acyl azide) + NaCl
RCOOC2H5 → RCONHNH2 (Acyl hydrazide) → RCON3 + 2H2O
Claisen Rearrangement
The classical Claisen rearrangement is the first and slow step of the isomerization of allyl aryl ethers to ortho allylated phenols. A cyclohexadienone is formed in the actual rearrangement step which is a [3,3]-sigmatropic rearrangement. Three valence electron pairs are shifted simultaneously.
Cyclohexadienone, a non-aromatic compound, cannot be isolated and tautomerizes immediately to the aromatic and consequently more stable phenol.
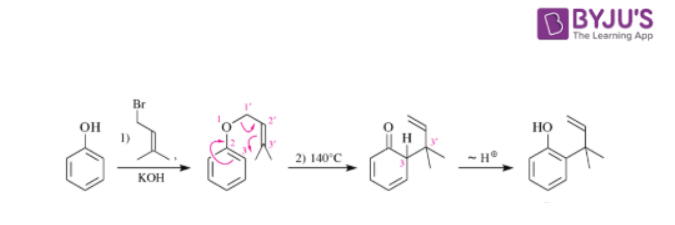
The Claisen rearrangement is a thermal rearrangement of allyl aryl ethers and allyl vinyl ethers respectively. It may be regarded as the oxa-version of the closely related Cope rearrangement. Claisen discovered this reaction first on allyl vinyl ethers and then extended to the rearrangement of allyl aryl ethers to yield o-allylphenols.
Beckmann Rearrangement
In the Beckmann rearrangement , an oxime is converted to an amide . An oxime is easily obtained by treatment of aldehyde or ketone with hydroxylamine. The OH group of ketoximes can become a leaving group. The Beckmann rearrangement of cyclic oximes results in lactams.
The comparison of the structure of the starting ketone with those of the products reveals that the combination of oxime formation and Beckmann rearrangement accomplishes the insertion of an NH group between the carbonyl carbon and the alpha carbon.
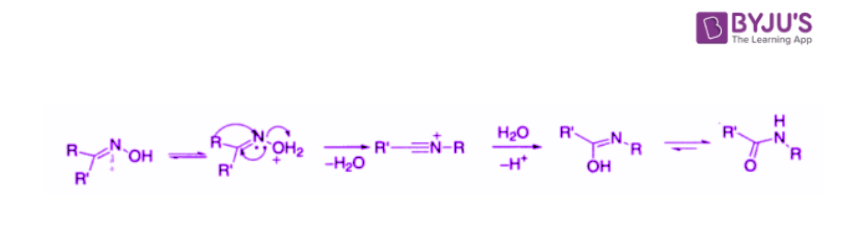
Beckmann rearrangement of the oxime of cyclohexanone is carried out on a very large scale industrially because the product, caprolactam, is the direct precursor of nylon 6, a versatile polymer that has many applications: for example, the manufacture of fibres for carpeting and other textiles. Concentrated sulphuric acid is used as both the acid catalyst and the solvent for the reaction.
Hofmann Rearrangement
The Hofmann rearrangement results from the treatment of a primary amide with bromine and hydroxide ion in water, ultimately forming an amine in which the carbonyl group of the starting amide has been lost.
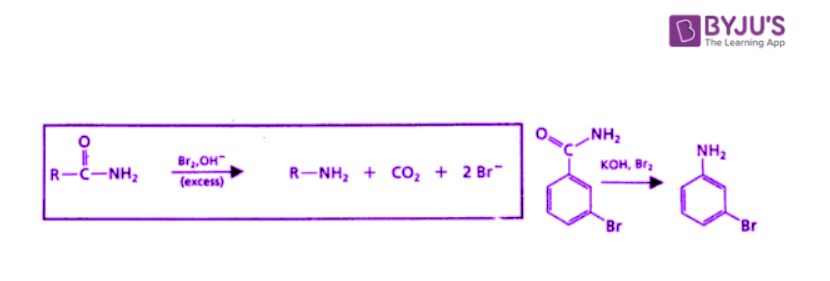
Thus, the Hofmann rearrangement results in a shortening of the carbon chain by one atom and a change in functional group from an amide to an amine. The Hofmann rearrangement occurs through a pathway similar to that for the Beckmann rearrangement.
Pericyclic Rearrangement
Pericyclic reactions are defined as the reactions that occur by a concerted cyclic shift of electrons. This definition states two key points that characterize a pericyclic reaction.
- First point is that reaction is concerted. In concerted reaction, reactant bonds are broken and product bonds are formed at the same time without intermediates.
- Second key point in pericyclic reactions involves a cyclic shift of electrons. The word pericyclic means around the circle. Pericyclic words come from the cyclic shift of electrons. Pericyclic reactions thus are characterized by a cyclic transition state involving the pi bonds.
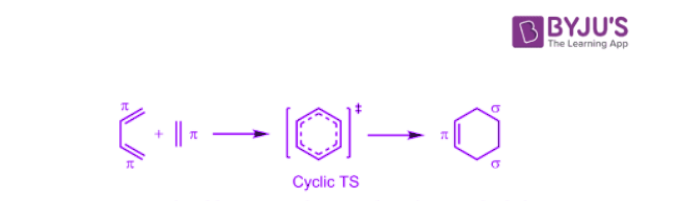
The energy of activation of pericyclic reactions is supplied by heat or by UV light. Pericyclic reactions are stereospecific and it is not uncommon that the two modes of induction yield products of opposite stereochemistry.
Three features of any pericyclic reaction are intimately interrelated. These are
- Activation – Pericyclic reactions are activated either by thermal energy or by UV light. However, many reactions that require heat are not initiated by light and vice versa.
- The number of pi bonds involved in the reaction.
- The stereochemistry of the reaction
Photochemical rearrangements
Many photoreactions are known to interconvert isomeric compounds. The term “rearrangement” is more general than “isomerization” but for the reactions under photochemical rearrangement will not be concerned with a distinction between these terms.
For convenience, we shall classify primary photochemical rearrangements as the following types.
- Cis trans isomerization
- Sigmatropic rearrangements
- Electrocyclic rearrangements
- Structural rearrangements which result from intramolecular cycloadditions.
In a broad sense, all four of these classes are special cases of pericyclic rearrangements and for concerted reactions, they all may be treated under a unifying framework guided by the rules derived from orbital symmetry considerations.
Frequently Asked Questions on Rearrangement Reaction
What is a rearrangement reaction? Explain with an example.
Usually, straight-chain alkanes are converted by heating in the presence of a catalyst to branched isomers. Examples include n-butane isomerization to isobutane and pentane to isopentane. Highly branched alkanes have favourable properties for internal combustion engines.
What is meant by Beckmann rearrangement?
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement to replace amides with an oxime functional group. The rearrangement of Beckmann is mostly catalyzed by acid.
What is a rearrangement in organic chemistry?
A rearrangement reaction is a large class of organic reactions, in which a molecule’s carbon skeleton is rearranged to give the original molecule a structural isomer. A substituent passes in the same molecule frequently from one atom to another.
What do you mean by the concerted path of a reaction?
A coordinated reaction is a chemical reaction in which all breaking of bonds and making of bonds occurs in one single step. There are no reactive intermediates or other high-energy unstable intermediates involved. The reaction, in which all bonds, are formed and broken in concerted process, is said to progress through a coordinated process.
What is a regioselective reaction?
Regioselectivity is the advantage of having or breaking a chemical bond in one direction over all other possible directions. Regioselectivity can also be extended to different reactions, such as pi-ligands addition. Selectivity occurs also in carbene insertions, e.g. in the Baeyer-Villiger reaction.

Comments