What are amines?
Amines are organic compounds and functional groups which contain a basic nitrogen atom with a lone pair of electrons. They are derivatives of ammonia where one or more hydrogen atom is replaced by substituents such as an alkyl or aryl group. They play a very significant role in the survival of life as they are involved in making amino acids. Amino acids are the building blocks of proteins in human beings. Amines act as nucleophiles due to the presence of lone pair of electrons in the nitrogen atom. They are involved in reactions such as acylation, electrophilic substitution, alkylation etc.
Electrophilic substitution reaction
The replacement of an atom or group of atoms through an electrophile is known as an electrophilic substitution reaction.
For example, bromination, nitration, sulphonation etc. of anilines comes under electrophilic substitution reaction.
In anilines, the maximum electron density is found at ortho- and para- positions of the –NH2 group. The -NH2 group is ortho and para directing and is a powerful activating group. The following significant reactions take place under this category:
Bromination
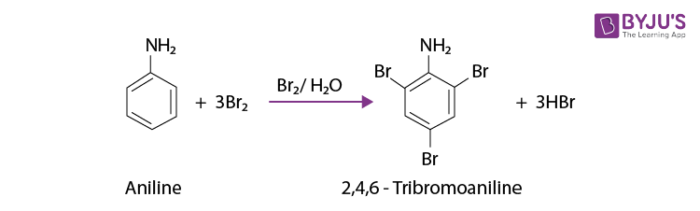
The reaction of aniline with bromine water at room temperature produces a white precipitate of 2,4,6 – tribromoaniline.
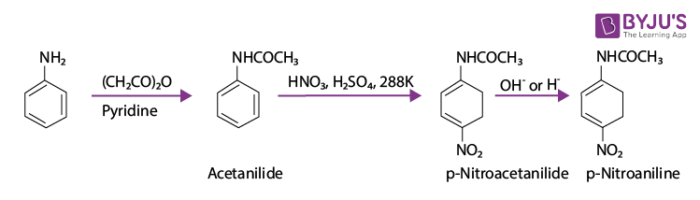
During the electrophilic reactions, the main problem encountered is the high reactivity of aromatic amines. Substitution occurs at ortho and para positions. If only a mono substituted aniline derivative is required, then the activating effect of the –NH2 group can be controlled by protecting the –NH2 group by acetylation with acetic anhydride. The desired substitution reaction is then carried out followed by the hydrolysis of substituted amides to substituted amines. The chemical equation for the same is shown below:

The lone pair of electrons present on nitrogen of acetanilide interacts with the oxygen atom due to resonance. Hence, the lone pair of electrons on nitrogen is less available for donation to the benzene ring. Therefore, activating effect of –the NHCOCH3 group is less than compared to that of amino group.
Nitration
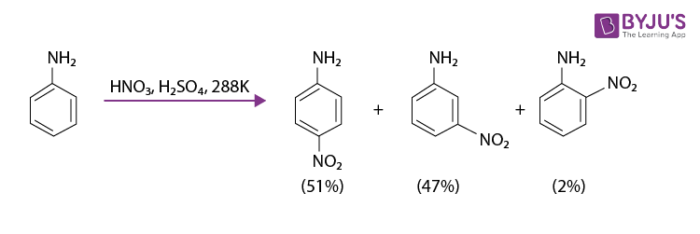
If aniline is subjected to direct nitration it yields tarry oxidation products in addition to nitro derivatives. In a strongly acidic medium, aniline is protonated to form anilinium ion which is meta directing. This is why apart from ortho and para derivative, a significant amount of meta derivative is also formed.
By protecting the –NH2 group by acetylation reaction with acetic anhydride, the nitration reaction is controlled. The p-nitro derivative is obtained as the major product in this case.
Sulphonation
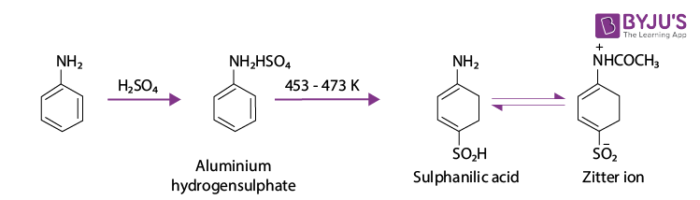
The reaction of aniline with concentrated sulphuric acid forms anilinium hydrogensulphate, which on heating with sulphuric acid at 453 – 473 K produces p-aminobenzene sulphonic acid which is commonly known as sulphanilic acid.
Aniline does not undergo Friedel- Crafts reaction due to the salt formation with aluminium chloride which acts as a catalyst. Due to this reaction nitrogen acquires a positive charge and hence acts as a strong deactivating group for further reactions.
This article covers the basic details of electrophilic substitution reactions in amines. For any further detail install BYJU’S – the learning app.

Comments