What are Colloids?
A colloid is a heterogeneous mixture in which the minute particles of one substance are dispersed in another substance, called the dispersion medium.
The minute particles here are 1 to 1000 nanometers in diameter, but they still remain suspended and do not settle at the bottom of the mixture. Sol, aerosol, foam, emulsion, etc., are some types of colloids. In this section, we will see how colloids are present in our everyday items of use and their applications in different fields.
Table Of Contents
- Examples of Colloid
- Commercial Applications of Colloid
- Recommended Videos
- Frequently Asked Questions – FAQs
Examples of Colloid
- Most of the food products which we eat today are colloids, including the dairy products. Cake, bread, milk, cream, butter, ice cream, margarine, fruit juices, whipped cream, etc. are colloidal in nature.

- The natural phenomena which we observe such as fog, mist, clouds, and rain are colloids in different forms. Even dust and smoke are colloidal.
- The blue colour of the sky can be credited to the suspended dust and water particles in the air which scatter blue light more than any other wavelength. Similarly, seawater is blue because of the colloidal impurities present in it, which also scatter blue light.
- A colloid is present in the fertile soil in the form of clay and humus material. It also plays a vital role in the storage and exchange of minerals.
Commercial Applications of Colloid
- A colloid is used as thickening agents in industrial products such as lubricants, lotions, toothpaste, coatings, etc.
- In the manufacture of paints and inks, colloids are useful. In ball-point pens, the ink used is a gel (liquid-solid colloid).
- The suspended impurities contained in the natural water are removed by adding sulfates of aluminium (alum) and of iron which coagulates them.
- Most of the medicines are colloidal. Colloidal gold and calcium are injected into the human body for the vitality of the muscles. Argyrol (silver sol) is used as an eye lotion. Albumin, Hetastarch, and Dextran are a few other colloids used in medicine.
Recommended Videos

Frequently Asked Questions – FAQs
What is a colloidal solution?
Colloids (also known as colloidal solutions or colloidal systems) are mixes in which insoluble particles of one substance are suspended in another substance at a microscopic level. Colloids are made up of chemicals that are uniformly distributed in one another.
What do colloids explain?
Colloids are mixes in which one or more chemicals are scattered throughout a solid, liquid, or gaseous medium as relatively large solid particles or liquid droplets. A colloid’s particles are generally electrically charged and remain scattered and do not settle owing to gravity.
How do you identify a colloid?
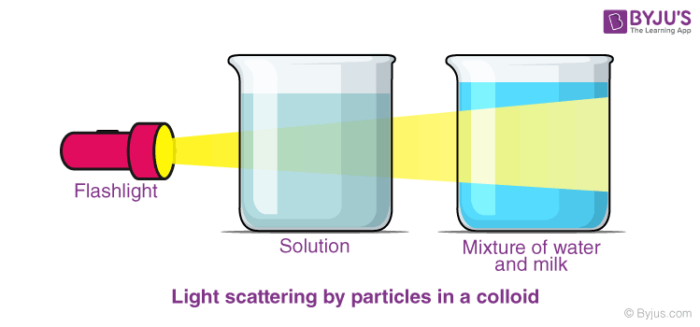
The Tyndall effect can be used to distinguish a colloid mixture from a solution. This is the point at which you shine a light through the mixture. When light bounces off the particles, the light shines through, indicating that you have a colloid combination.
What are two examples of colloids?
A colloid is a type of solution in which the size of the solute particles is somewhere in between real solution and suspension. Mayonnaise, milk, butter, gelatin, and jelly are examples of colloids.
Are colloids homogeneous mixtures?
Colloidal particles are large molecules or groups of smaller species that scatter light. On a macroscopic (visual) size, colloids are homogeneous, whereas solutions are homogeneous on a microscopic (molecular) scale.
To learn more about colloids, their classification and applications, download BYJU’S – The Learning App.

Comments