What is Bromothymol blue?
Bromothymol Blue is an indicator in the pH range from 6.0 to 7.6.
Bromothymol blue is the most commonly used pH indicator and is in low concentration and size container and low toxicity. It contains a pH indicator, bromothymol blue, which causes the medium to change from yellow to blue-green; it contains antimicrobial agents, chloramphenicol and chlortetracycline, and an antimycotic agent, cycloheximide.
Other name – Dibromothymolsulfophthalein, 3,3′-Dibromothymolsulfonphthalein
| C27H28Br2O5S | Bromothymol Blue |
| Density | 1.25 g/cm³ |
| Molecular Weight/ Molar Mass | 624.384 g/mol |
| Boiling Point | 614.26° C at 760 mmHg |
| Melting Point | 202 °C |
| Chemical Formula | C27H28Br2O5S |
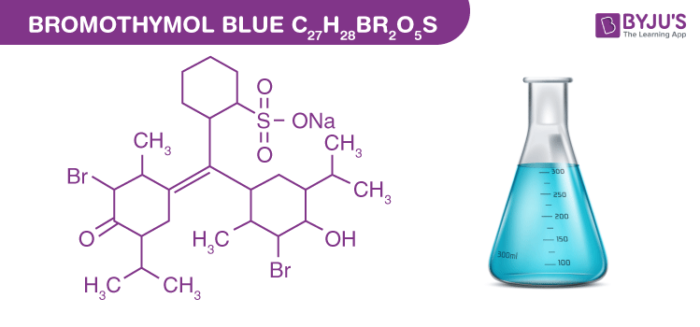
Bromothymol blue Structure – C27H28Br2O5S
Physical Properties of Bromothymol blue – C27H28Br2O5S
| Odour | Odourless |
| Appearance | Blue in basic solutions, yellow in acidic solutions and green in neutral solution |
| Covalently-Bonded Unit | 1 |
| Complexity | 818 |
| pH | 7.6 |
| Solubility | Sparingly soluble in water |
Uses of Bromothymol blue – C27H28Br2O5S
- In conjunction with phenol red, bromothymol blue was used to monitor the activity of the fungal asparaginase enzyme with phenol turns to pink and bromothymol blue turns blue indicating an increase in pH and thus enzyme activity.
- In obstetrics, bromothymol is used to detect premature membrane rupture. Typically, amniotic fluid has a pH > 7.2, so bromothymol turns blue when it comes into contact with amnion – leaking fluid.
Frequently Asked Questions
What colour is Bromothymol blue when in a base?
Blue bromthymol is a mild acid. Based on the solution’s pH it can be in acid or base form. For acidic solutions, this reagent is purple, blue for simple solutions and green in neutral solutions.
What does Bromothymol blue indicate the presence of?
Bromothymol blue (BMB) is an indicator dye which turns yellow when an acid is present. When carbon dioxide is added to the solution, carbonic acid is produced which lowers the solution’s pH.
What happens when you drink Bromothymol blue?
Bromothymol blue powder can cause irritation of the skin and eyes, and acute toxicity if swallowed. Tiny, spontaneous digestion has not seen any problems, but high levels can cause nausea and vomiting, even death. Drinking water will treat incidental swallowing.
Why is Bromothymol blue-green in neutral solutions?
Bromothymol blue serves in the solution as a heavy acid. In neutral water, this is bluish-green. The deprotonation of the neutral form results in a highly conjugated structure which accounts for the color difference. The greenish colour of the neutral solution is responsible for an intermediate of the deprotonation process.
Is Bromothymol Blue toxic?
Chronic effects on humans: causes the following organs to suffer damage: lungs, mucous membranes. Many Adverse Effects on Humans: Very dangerous for ingestion. Dangerous for skin touch (irritant), and inhalation.

Comments